Researchers found clinical benefits of a chimeric antigen receptor (CAR) T-cell therapy, which obtained approval with objective response rate and complete response data only, without a randomized, controlled study.
Gilead Sciences’ Yescarta (axicabtagene ciloleucel) demonstrated a high ORR and a 73 percent reduction in the risk of death in refractory large B-cell lymphoma (LBCL), compared to salvage chemotherapy, a study said.
Blood Advances, published by the American Society of Hematology (ASH), released the results of the study, titled, “Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs. salvage chemotherapy in refractory large B-cell lymphoma.”
The research team said survival rates of LBCL, the most common subtype of non-Hodgkin lymphoma, have improved in the past few decades, but the prognosis of relapsed or refractory LBCL remained poor, and there were few treatment options.
Only 30 to 40 percent of patients whose disease progresses during initial immunochemotherapy or within one year after the first remission will respond to salvage chemotherapy and be able to receive autologous stem cell transplant (ASCT) subsequently, the research team said.
Amid few treatment options for refractory LBCL, recently developed CAR-T cell therapy showed significant efficacy.
Yescarta, used in this study, showed 83 percent ORR and 58 percent CR in patients who progressed after chemotherapy and transplantation or who are refractory and obtained the marketing approval in the U.S. and Europe.
However, as a CAR T-cell therapy is customized for one individual patient, it was difficult to know its clinical benefits compared to salvage chemotherapy used in clinical patient care.
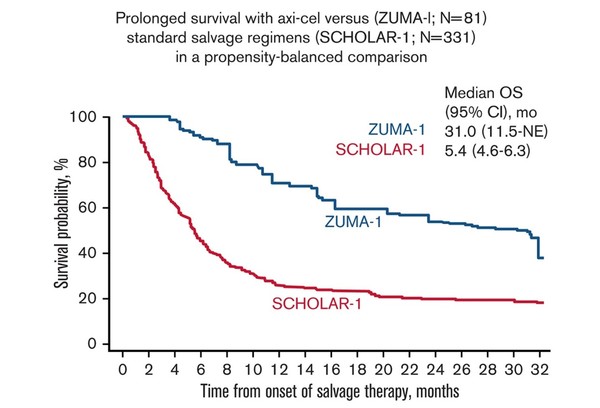
So, the research team compared the global retrospective SCHOLAR-1 study, which demonstrated the prognosis of salvage chemotherapy in refractory LBCL, with the two-year outcome of the Phase 1/2 ZUMA-1 study, which was the basis for approval of Yescarta.
First, the research team used propensity scoring to adjust the balance between ZUMA-1 and SCHOLAR-1 patients.
Then, the team analyzed the response and survival data of 101 patients who participated in the phase 2 portion of the ZUMA-1 study. In SCHOLAR-1, they evaluated response in 434 patients and survival in 424 patients.
The results showed that patients in ZUMA-1 showed a significant improvement in response and survival than SCHOLAR-1 patients, even though ZUMA-1 patients were more heavily pretreated.
During the median follow-up of 27.1 months in ZUMA-1, the ORR and CR were 83 percent and 54 percent, respectively. In SCHOLAR-1, the ORR and CR recorded 34 percent and 12 percent.
The two-year survival was 54 percent in ZUMA-1, and 20 percent in SCHOLAR-1, indicating that patients treated with Yescarta had a 73 percent lower death risk than those treated with salvage chemotherapy.
“These results were consistent with those of an additional standardization analysis in which strata were limited to two prognostic factors (refractory categorization and presence/absence of stem cell transplant after refractoriness to chemotherapy) to conserve sample size,” the research team said.
Although the study had a limitation of a non-randomized analysis, the results mean that Yescarta offered durable responses and a substantial survival benefit, compared to non-CAR T-cell salvage treatment for patients with refractory LBCL, it added.

