An FLT3 inhibitor HM43239, developed by Hanmi Pharmaceutical and licensed out to Aptose Biosciences recently, reported excellent tolerability and anticancer activity in all patients with relapsed or refractory FLT3 mutated and wild-type acute myeloid leukemia (AML), a study showed.
At the annual meeting of the American Society of Hematology on Dec. 13 (ASH 2021), Dr. Naval Daver at MD Anderson Cancer Center will give an oral presentation on the data of the Phase 1/2 trial of HM43239 in the Acute Myeloid Leukemia session.

HM43239 inhibits FLT3 mutation (FMS-like tyrosine kinase 3 ITD and TKD) and spleen tyrosine kinase (SYK) related to resistance to FLT3-targeting treatment.
The investigational drug obtained orphan drug designation (ODD) from the U.S. FDA in 2018 and a clinical-stage orphan drug status from Korea’s Ministry of Food and Drug Safety in 2019.
The drug recently drew attention after Hanmi licensed it out to Aptose Biosciences in a $420 million deal.
According to the abstract of the study released by the ASH before the annual meeting, the study includes data of 28 patients enrolled for phase 1 trials between March 2019 and June 2021.
Thirteen were in the dose-escalation cohorts (20-160mg) and 15 in the dose-expansion cohort at 80mg.
The patients had an average of two prior treatments, including existing FLT3 inhibitors such as gilteritinib. Ten patients (36 percent) had FLT3 mutation, 16 (57 percent) had FLT3 wild-type, two (7 percent) were FLT3 unknown at the time of enrollment.
The most frequently reported FLT3 mutation types were ITD (21 percent), followed by TKD (11 percent), and ITD/TKD (4 percent).
The most common drug-related treatment-emergent adverse events (TEAEs) were diarrhea (14 percent), nausea (7 percent), vomiting (7 percent), and alanine aminotransferase (ALT) increased (7 percent). No TEAEs exceeding Grade 3 were observed until the data cut-off time.
The study showed that five (17.9 percent) of the 28 patients achieved complete remission (CR) after receiving 80mg of HM43239. The composite CR was 26.3 percent in the 80mg dose group (19 patients).
Eight patients in the 80mg dose group had FLT3 mutation, and three achieved CR after HM43239 treatment. One of the three had been previously treated with gilteritinib (brand name: Xospata).
As gilteritinib is a targeted therapy for relapsed or refractory FLT3 mutated patients, the results indicate that HM43239 could be used for gilteritinib-resistant patients.
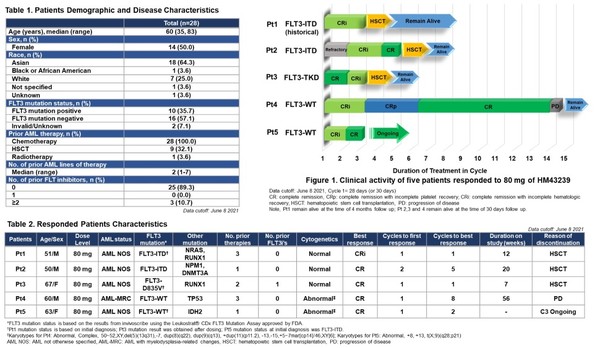
Also, in the 80mg dose group, the other 11 patients were FLT3 wild-type, and two of them (18 percent) achieved CR after HM43239 treatment.
One of the five patients who had CR had a relapse of AML due to TP53 mutation, but three received hematopoietic stem cell transplantation and have remained alive to date. The other patient remained in CR in cycle three.
The plasma concentration showed a dose-dependent increase after one dose of 20 to 160 mg. After 17 days of treatment, it reached a stable state. The plasma inhibitory activity (PIA) assay showed dose-dependent inhibition of pFLT3 with up to 90 percent at the dose levels of 80 mg or more.
“HM43239, a preclinically potent FLT3 and SYK inhibitor, showed a favorable safety profile with only mild adverse events and no dose-limiting toxicities (DLTs) in this ongoing phase 1/2 study,” the research team said. “At 80 mg dose, HM43239 demonstrated clinical activity in both FLT3 mutated and FLT3 wild-type AML.”
The research team said it would continue to evaluate doses above 80mg to determine the optimal recommended phase 2 dose.
The research team was enrolling the dose-escalation cohort of 160mg and the dose-expansion cohort of 120mg and will present updated response, safety, and pharmacokinetic data, it added.

