Hemophilia patients often call Sanofi’s fitusiran “a dream treatment” because the single subcutaneous injection once a month shows a preventive effect.
The investigational drug proved its prophylaxis effect in patients with hemophilia A or B with inhibitors in a recent study, drawing much attention.
However, the drug’s safety issue related to blood clotting, which caused the suspension of the clinical trial of fitusiran, remains a challenge, observers said.
Researchers will present the results of the phase 3 ATLAS-INH study of fitusiran at the Plenary Scientific Session during the annual meeting of the American Society of Hematology in Atlanta on Dec. 11-14.
The ATLAS-INH trial is an open-label, randomized and comparative trial that compares once monthly 80 mg subcutaneous fitusiran with on-demand bypassing agents (BPA) in patients with hemophilia A or B with inhibitors who are treated with on-demand BPAs in bleeding events.
Some hemophilia patients develop inhibitors to commonly used coagulation factor agents, making it difficult to stop bleeding events. For this reason, patients with inhibitors get BPAs in bleeding episodes. Helimbra (ingredient: emicizumab) is available as preventive therapy for hemophilia patients with inhibitors.
However, Helimbra is a subcutaneous injection once to four times monthly, and the treatment can be used only for hemophilia A.
Fitusiran is a small interfering RNA (siRNA) treatment targeting antithrombin to restore thrombin generation and rebalance hemostasis. It can be used in all patients with hemophilia, regardless of type A or type B, and with or without inhibitors.
The study results showed that fitusiran significantly reduced annualized bleeding rate (ABR), set as the primary endpoint. In addition, compared to the control group, the therapy also improved spontaneous ABR, joint ABR, and quality of life (QoL) measured by Haem-A-QoL.
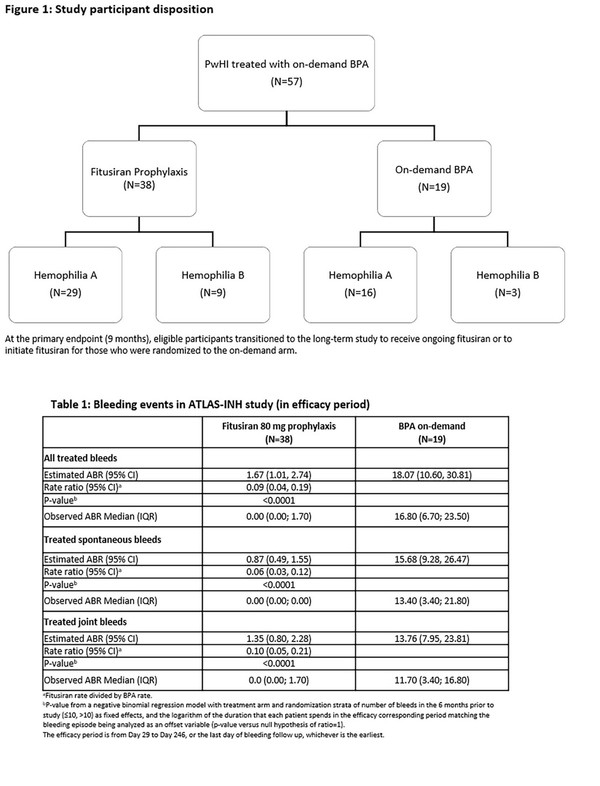
A total of 57 patients were assigned randomly to the fitusiran and control groups, and their mean age was 28.4 years.
In the fitusiran group, 65.8 percent (25 patients) did not report any bleeding event, and the prophylaxis effect was observed in all hemophilia A and hemophilia B patients.
The fitusiran group and the control group also showed statistically significant differences in physical health domain score.
Treatment-emergent adverse events (TEAEs) were reported in 92.7 percent (38 patients) of the fitusiran group and 57.9 percent (11 patients) of the BPA group. Among them, seven (17.1 percent) from the fitusiran group reported 13 serious adverse events, and five (26.3 percent) in the control group, eight.
In the fitusiran prophylaxis arm, adverse reactions included device-related infection, hematuria, spinal vascular disorder, subclavian vein thrombosis, thrombosis, acute cholecystitis, chronic cholecystitis, and asymptomatic Covid-19.
One patient (2.4 percent) experienced side effects of spinal vascular disorder and thrombosis that led to treatment discontinuation. There were no fatal TEAEs.
“This phase 3 study demonstrated the efficacy of the 80 mg monthly subcutaneous prophylaxis dose of fitusiran in people with hemophilia A or B with inhibitors,” the research team concluded. “Specifically, fitusiran significantly reduced bleeding with a median ABR of zero and a significant proportion of people with zero bleeds, resulting in a meaningful improvement in health-related quality of life.”
The research team went on to say that serious adverse events were consistent with the previously identified risks of fitusiran. It added that a different fitusiran dosing regimen with reduced dose and dose frequency is being tested in ongoing clinical trials.
The safety issue of fitusiran has been repeatedly pointed out since early-stage clinical trials.
In 2017, a phase 2 trial of fitusiran was suspended after a patient died of cerebral hemorrhage and was resumed with a revised study design later that year.
In October last year, Sanofi voluntarily paused a phase 3 study after finding non-fatal levels of thrombotic symptoms in a trial participant. Later, Sanofi analyzed data with the U.S. FDA and resumed fitusiran dosing.
That explains why whether the latest study results will accelerate fitusiran development draws attention, industry watchers said.

