Roche Korea said it would actively expand new drugs in Korea at an online press conference, marking the 125th anniversary of the foundation of Roche Group.
With the conference title, “We are Roche – The Future of Healthcare,” the company presented Roche Grou’s achievements in the past 125 years, visions for the future, a 10-year plan, and R&D pipelines.

Lee Seung-hun, Medical Partnership Cluster Lead at Roche Korea, shared Roche Group’s R&D pipelines and a plan to build a Korean-specific healthcare system.
According to Lee, Roche Group has ranked first in R&D investment among pharmaceutical companies since 2011. Based on the robust R&D, the company is focusing on developing new pipelines in cancer and infection, ophthalmology, nervous system, and rare diseases that remain unexplored.
“Through massive R&D investment, which accounts for over 20 percent of sales, Roche Group is reinforcing its ability to develop new drugs not only for cancer but rare and intractable diseases where treatment options are limited,” Lee said.
Roche’s R&D is also active in Korea, he noted.
Roche Korea is supporting Korean patients to participate in multinational clinical trials. In addition, the company signed an MOU with the government and related societies in 2020 to invest 170 billion won ($144.3 million) for the next five years to help establish a customized healthcare environment, according to Lee.
Despite the Covid-19 pandemic, Roche obtained approval for 25 new clinical trials. Lee said that the company is conducting over 400 studies to develop new drugs as of the first half of this year.
“Over 40,000 patients have participated in Roche’s clinical trials in Korea. But, unfortunately, most of them did not have many treatment options. So it is meaningful that we provided treatment opportunities for them.”
Lee said that the number of Korean patients registered for Roche’s clinical trials was the world’s fourth-largest as of the first half of this year.
Roche’s plan to commercialize investigational drugs in Korea is progressing as scheduled, he went on to say.
“Along with new drugs in neurology and ophthalmology, we will make efforts to introduce Roche’s new immunotherapy Tiragolumab, Alzheimer’s disease drug Gantenerumab quickly in Korea,” he said.
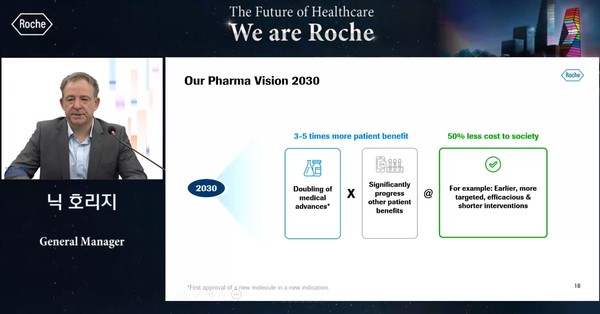
Nic Horridge, General Manager of Roche Korea, also said he would endeavor to raise Korean patients’ accessibility to Roche’s novel drugs.
Since taking office, Horridge has been aggressively working to provide broader accessibility for Korean patients to use new drugs such as Tecentriq, he said. “I will continue these kinds of efforts.”
Established in 1983, Roche Korea introduced targeted therapy, immunotherapy, and anticancer drugs that can be used regardless of cancer type.
Also, the company is running patient-centered social contribution programs such as “Healing Together” to support culture and art activities for rare and intractable disease patients and “Volunteer Together,” where employees volunteer for patients and the underprivileged.

