Korean healthcare industry-related investors and consultants said the Korean digital therapeutics (DTx) market is likely to achieve robust growth with continued investment, despite challenges to overcome regulations and security issues.
On Wednesday, the experts made such remarks at an online forum hosted by Samsung Medical Center’s Smart Healthcare Research Institute and Digital Therapeutics Research Center to celebrate winning the government’s “K-DEM (Digital and Electronic Medicine) Station” project.
Kim Young, CEO of Synex, a consulting firm for healthcare companies, spoke on the status and prospects of the digital healthcare device industry.
Noting that a wide range of DTx products is being developed in the U.S. and Europe, they will be used in various fields, including data collection and analysis, improvement of medication adherence, cognitive behavioral therapy, and chronic disease management, Kim said.
In other countries, DTx is actively used for diabetes and obesity control and lifestyle management, and the DTx market size keeps growing, too, she said.
According to a recent Health Insurance Review and Assessment Service (HIRA) report, the global DTX market grew 23.1 percent to $3.5 billion in 2020. By 2028, the market size will reach $19.1 billion, the report predicted.
In Korea, biotech firms are conducting trials of DTx for cognitive therapy, insomnia treatment, and myopia in children.
AIMMED and WELT have begun testing DTx for insomnia in clinical trials.
In September, LifeSemantics won the regulatory nod for a confirmatory trial of respiratory rehabilitation software Redpill Breath.
“There are still challenges such as how to differentiate DTx from wellness apps and how to persuade patients to keep participating,” Kim said. “Encouraging clinicians to use DTx will be a force to build the DTx industry.”
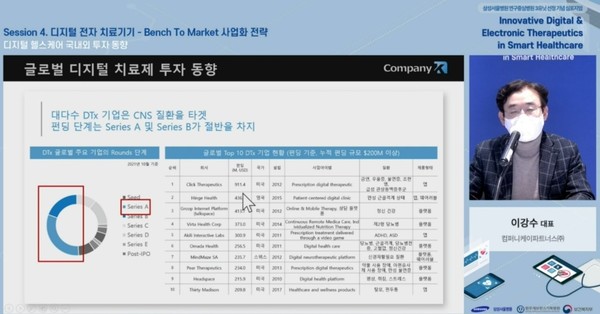
Company K Partners CEO Lee Kang-soo also raised expectations for DTx industrial growth.
According to Lee, over 40 DTx products won FDA approval as of June. In addition, he said the overall funding increased about 1.5 folds compared to last year.
“The Korean government seems to have a strong will to revitalize the DTx market as it designated DTx as a new growth engine and made regulations and guidelines,” Lee said.
On Dec. 7, the Ministry of Food and Drug Safety published guidelines for evaluating DTx products’ safety and performance and clinical trial plans, saying the government would support the fast commercialization of DTx.
guidelines contain safety and performance evaluation standards and methods for DTx in insomnia and alcohol/nicotine addiction disorders and clinical design methods and efficacy performance standards for DTx.
Korea’s major conglomerates are entering the digital healthcare sector, and Naver, the nation’s largest internet portal, established a healthcare research center, Lee said. “Venture capital firms are highly interested in the DTx sector, and many venture funds are being formed. As a result, there will be larger venture investment from now on.”

