Daewoong Pharmaceutical is seeking to expand the indication of Easyef Oint., a wound ointment containing epidermal growth factor (EGF), to treat adverse skin reactions in cancer patients shown after the use of epidermal growth factor receptor (EGFR) inhibitors such as erlotinib.
On Monday, the Ministry of Food and Drug Safety approved Daewoong’s plan to conduct a phase 2 clinical trial of DWP708.
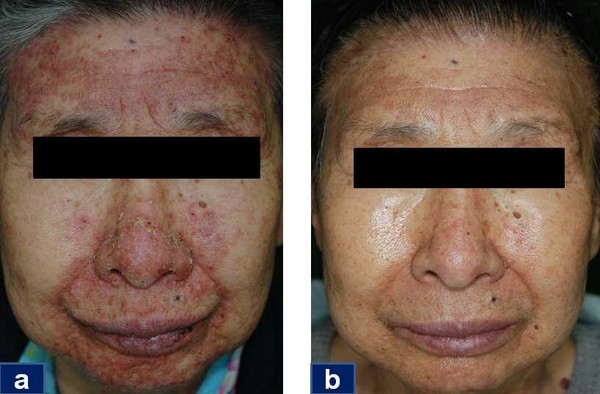
The phase 2 study aims to explore DWP708’s efficacy in EGFR inhibitor-related skin side effects and evaluate the drug’s safety. The multicenter, randomized, double-blind, and placebo-controlled study will occur at Gyeongsang National University Hospital and Dong-A University Hospital.
In 2010, Daewoong released Easyef, an over-the-counter adjuvant treatment for wounds and skin ulcers. From October 2012 to November 2013, Daewoong conducted a non-clinical phase 2 comparison study in 52 patients with non-small cell lung cancer (NSCLC) treated with erlotinib alone or with pancreatic cancer treated with gemcitabine plus erlotinib.
Through the study, Daewoong concluded that its EGF ointment was effective on erlotinib-related skin effects (ERSE) regardless of sex, age, tumor type, and dose of erlotinib and that the treatment improved all types of ERSE symptoms evenly.
Based on this study, Daewoong won the patent of the ointment’s pharmaceutical composition from the Korea Intellectual Property Office in 2015.
The name of the patent is “Pharmaceutical Composition for Preventing or Treating Skin Rash.” Patent holders are Daewoong Pharmaceutical and Dong-A University Industry-Academy Cooperation Foundation.
Daewoong said skin rash was a common side effect of all EGFR inhibitors in the patent statement.
In treating NSCLC and pancreatic cancer, the company said there was an industrial need to develop a method to suppress skin rash caused by EGFR inhibitors, including erlotinib.
“This invention provides a pharmaceutical composition including EGF as an active ingredient for preventing or treating skin rash, which is caused by the administration of EGFR inhibitors such as erlotinib,” Daewoong said.

