BMS is getting ready to tap the psoriasis treatment market with a novel, oral, tyrosine kinase 2 (TYK2) inhibitor, which averted the safety risk of the existing Janus kinase (JAK) inhibitors.
At the 40th Annual JP Morgan Healthcare Conference, BMS introduced the first TYK2 inhibitor deucravacitinib, which the company is developing as one of the future growth engines.
The company has applied for marketing approval for psoriasis treatment in the U.S., Europe, and Japan. The company said the U.S. is expected to grant authorization around Sept. 10.
If deucravacitinib obtains the nod for psoriasis treatment, it will become the first-in-class TYK2 inhibitor.
Since the time of R&D of the TYK2 inhibitor, the company has emphasized the difference between the TYK2 inhibitor and other JAK inhibitors that are being rolled out as autoimmune disease treatment.
TYK2 is also known as a JAK family, along with JAK1, JAK2, and JAK3.
To distance itself from the safety issue of JAK inhibitors like Xeljanz (topacitinib), BMS is stressing that its TYK2 inhibitor has a different mechanism of action.
Researchers who participated in the development of deucravacitinib said in their study that TYK2, an intracellular signaling enzyme, was responsible for STAT (signal transducer and activator of transcription)-dependent gene expression and activating the functional response of interleukin-12 (IL-12), interleukin-23 (IL-23), type I and III interferon receptors.
“These cytokine pathways are involved in the pathological processes associated with immune-mediated disorders, including psoriasis, and are distinct from responses induced by JAK1, JAK1, and JAK3 combinations, JAK2, or other signaling kinases,” the study said.
In other words, the TYK2 inhibitor with selective cytokine pathways can be clinically effective in treating autoimmune and inflammatory diseases and avoid some complications reported in non-selective JAK inhibitors.
In the phase 3 POETYK PSO-1 and POETYK PSO-2 trials, deucravacitinib proved its efficacy and safety in patients with moderate-to-severe plaque psoriasis compared with placebo and active drug Otezla (apremilast).
“Deucravacitinib showed superiority to placebo and Otezla, showing clinically meaningful efficacy improvements in skin improvement, symptom burden, and quality of life measures,” BMS said.
“Deucravacitinib also clinically lowered adverse laboratory reactions such as hematologic adverse events and showed excellent tolerability with a low rate of treatment discontinuation.”
BMS sold Otezla, a selective PDE4 inhibitor, to get antitrust clearance when it acquired Celgene in early 2019. Amgen purchased it and successfully released it in the market.
At the time, BMS kept deucravacitinib while selling Otezla. Finally, Amgen purchased Otezla and grew it as a blockbuster drug.
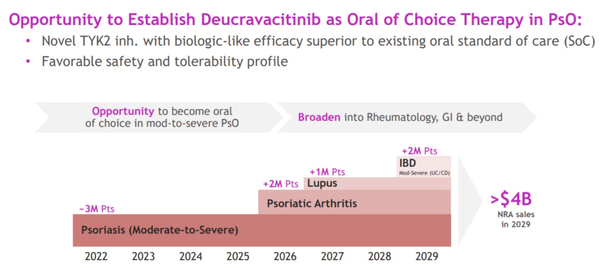
At the JP Morgan Healthcare Conference, BMS said that deucravacitinib is expected to generate $4 billion annual revenue by 2029.
BMS announced that starting with the psoriasis market in September this year, it plans to expand the indication of deucravacitinib to psoriatic arthritis in 2025, lupus in 2026, and inflammatory bowel disease in 2028.

