Recently, CAR-T cell therapies such as BMS’ Breyanzi (lisocabtagene maraleucel) and Gilead’s Yescarta (axicabtagene ciloleucel) proved effective in the early treatment of relapsed/refractory diffuse large b-cell lymphoma (DLBCL), signaling a possibility to replace autologous hematopoietic stem cell transplant (auto-HCT).
However, a study said that chemotherapy-sensitive patients with DLBCL should maintain auto-HCL as the standard of care rather than getting CAR-T.
The research team of Dr. Mazyar Shadman at the Clinical Research Division of Fred Hutchinson Cancer Research Center in Seattle, the U.S., published the study, titled, “Autologous transplant vs. chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission,” in the March issue of Blood, a journal of the American Society of Hematology (ASH).
The research team used the Center for International Blood & Marrow Transplant Research (CIBMTR) registry database to identify patients with DLBCL who had a partial response after salvage chemotherapy and received either an auto-HCT (2013-2019) or Yescarta (2018-2019).
Then, they compared the clinical results between the two cohorts using univariable and multivariable regression models after adjustment for relevant baseline and clinical factors.
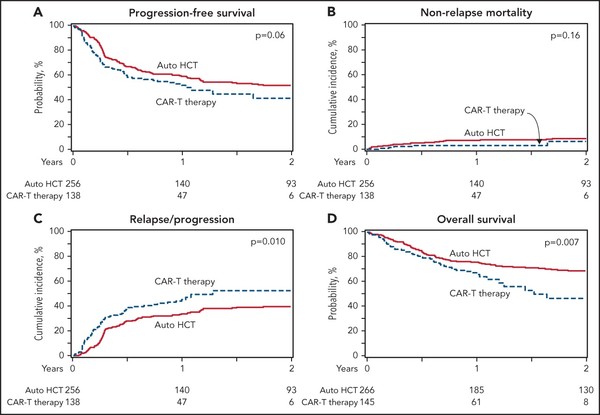
In the univariable analysis, the two cohorts showed no difference in two-year progression-free survival (PFS) and the rate of 100-day non-relapse mortality.
However, consolidation with auto-HCT was associated with a lower rate of relapse/progression and a superior overall survival (OS) at two years.
In the multivariable regression analysis, the auto-HCT group had a significantly lower relapse/progression rate risk and a superior OS.
“In patients with DLBCL in a partial response after salvage therapy, treatment with auto-HCT was associated with a lower incidence of relapse and a superior OS compared with CAR-T,” the research team said. “These data support the role of auto-HCT as the standard of care in transplant-eligible patients with relapsed DLBCL in partial response after salvage therapy.”
Premal D. Lulla, an assistant professor at Baylor College of Medicine, supported Shadman‘s study by publishing an article, “CAR T cells and autologous transplantation can coexist for DLBCL,” in the same journal.
Although two trials of Breyanzi -- TRANSFORM and ZUMA7 – proved that CAR-T cell therapies were superior in event-free survival in the second-line setting for DLBCL treatment, compared with current standard treatments, the two studies did not allow salvage chemotherapy, Lulla noted.
Thus, he said the two studies are not enough to represent all the patients that doctors see in practice.
Citing the BELINDA study of Novartis’ Kymriah (tisagenlecleucel), whether to allow salvage chemotherapy before CAR-T cell therapy affected the results of clinical trials, Lulla said.
“Once CAR-T cells are approved as second-line treatment in DLBCL, a randomized trial including only chemosensitive patients with DLBCL is unlikely to be conducted, leaving a void to guide practice in this subset of patients,” he added.
Thus, Shadman’s study results will help to determine the treatment direction for patients, who are not in the same type of ZUMA-7 and TRANSFORM participants, he said.
Lulla said the largest difference between Shadman’s study and ZUMA-7/TRANSFORM was “chemosensitivity.” The former included patients who proved chemosensitivity, whereas the latter included the highest chemorefractory risk.
Also, in Shadman’s study, differing numbers of prior lines of therapy and disease burden at the time of treatment could indicate that CAR-T cell therapy recipients were more chemorefractory, Lulla said.
Shadman’s research team recognized these potential cofounders and matched disease burden, the median number of prior lines of therapy, and they still showed that auto-HCT was equivalent to CAR-T for DLBCL, he added.
“As CAR-T cells are now poised to overtake salvage chemotherapy and ASCT (autologous hematopoietic stem cell transplantation) in the second-line setting, at least for those with a chemorefractory disease, this timely report from Shadman et al. argues for maintaining the standard approach for those with chemosensitive DLBCL,” Lulla said.

