Olympus Korea held a news conference on Thursday to announce the market release of EndoBRAIN-EYE, an AI-backed colonoscopy diagnosis support system.
However, the new AI technology has to overcome many challenges to be used by clinicians in the field, industry watchers said.

EndoBRAIN-EYE is assistant software that helps physicians diagnose legions through deep learning based on about 3.95 million colonoscopy images and quantitatively analyze them.
If a legion such as a polyp or a tumor is detected during a colonoscopy, a notification sound goes off, and colors are displayed around the legion.
Olympus Korea emphasized that EndoBRAIN-EYE demonstrated 98 percent sensitivity and 93.7 percent specificity in a clinical trial.
The software can be used with Olympus’ gastrointestinal endoscope EVIS LUCERA ELITE and EXERA III models. Medical institutions equipped with those products can install the software and use it.
However, it is questionable whether clinicians would be willing to pay the expense of installing EndoBRAIN-EYE, which costs millions of won.
When Olympus released EndoBRAIN-EYE in Japan in 2020, it set the price at about 3 million yen ($25,263). Olympus Korea has yet to determine the price in Korea.
The success of marketing EndoBRAIN-EYE hinges upon whether it will get health insurance benefits. Olympus Korea is also aware of this circumstance.
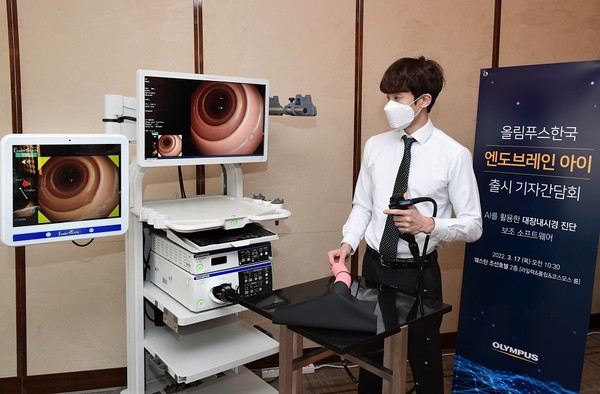
Park In-je, head of the gastrointestinal and respiratory business unit at Olympus Korea, said it usually takes some time for clinicians to use a medical device with innovative technology.
“External factors, particularly insurance policy, play a significant role. Olympus will make efforts to help shape the related market after launching the AI-backed system,” he said.
EndoBRAIN-EYE is not reimbursable in Japan, either, but the company is making multifaceted efforts to resolve this issue, he went on to say. “It’s the same in Korea. We will help make an environment to use AI-powered systems in endoscopy,” he added.
Many developers are working on new AI technologies for medical use, but no medical device has won reimbursement in Korea. This is because most AI-powered products have failed to secure evidence for cost-effectiveness superior to conventional technologies or overcome adverse reactions as much as the government desired. EndoBRAIN-EYE has yet to secure data that demonstrated better cost-effectiveness or shorter procedure time than existing procedures.
Park said he was aware of the matter and would search for various ways to secure evidence.
He predicted that Korean doctors would use innovative products such as EndoBRAIN-EYE.
“As many hospitals use Olympus’ gastrointestinal endoscopy products, these experiences will help the new product settle in the market. This will naturally lead us to secure data,” he said.

