LG Chem is gearing up to enter the Chinese market with a premium hyaluronic acid filler brand.
On Thursday, the Korean chemical company updated the status of the clinical trial of YVOIRE Y-Solution 360 to “recruiting,” according to ClinicalTrials.gov, a registry of clinical trials run by the U.S. National Institutes of Health.
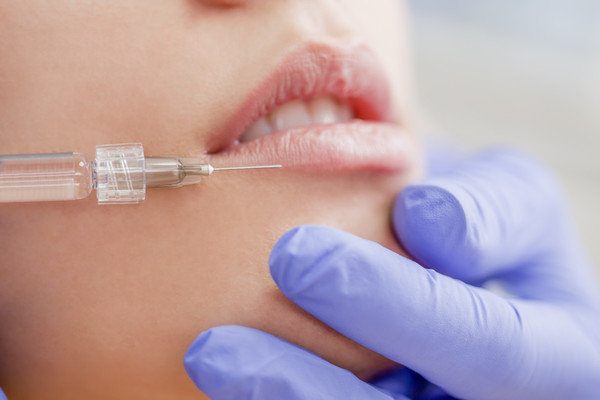
LG Chem began the study in January and recently started enrolling patients.
The trial will be multicenter, randomized, evaluator-blind, and no-treatment controlled.
The company plans to complete data collection by December 2023 and end the trial in August 2024.
YVOIRE Y-Solution is LG Chem’s premium dermal filler brand. It is categorized as YVOIRE Y-Solution 360, YVOIRE Y-Solution 540, and YVOIRE Y-Solution 720, depending on volume restoration.
In addition to the YVOIRE Y-Solution 360 study, LG Chem started trials in China for YVOIRE Y-Solution 540 (improving nasolabial folds) and YVOIRE Y-Solution 720 (middle facelift), respectively. The company is recruiting patients.
“The YVOIRE product lineup is tapping the Chinese market,” an official at LG Chem said. “To meet various customer needs, we are preparing for a premium brand’s entry into China.”
In July last year, LG Chem and Hangzhou Jiansheng Medicine established a joint venture, LG Jiansheng Life Science, to directly sell and distribute filler products. In addition, the company released a local filler brand as well.
LG Chem entered the Chinese market in 2013. Since 2016, the company has led the dermal filler market with about 25 percent of the market share. Moreover, if a premium filler lineup enters China, LG Chem will have better competitiveness, the company said.
“We expect to roll out YVOIRE Y-Solution in China in 2025,” an official at LG Chem said.

