Amgen’s first KRAS targeted therapy, Lumakras (ingredient: sotorasib), demonstrated consistent clinical benefits in the longest follow-up of patients with KRAS G12C-mutated non-small cell lung cancer (NSCLC).
On Sunday, Amgen released the two-year data of the CodeBreak100 phase 1/2 trial of Lumakras at the American Association for Cancer Research (AACR) annual meeting.
Lumakras is the first oral treatment that selectively inhibits the KRAS G12C mutation involved in the development of NSCLC.
In Korea, Lumakras obtained the license for adult patients with locally advanced or metastatic NSCLC with KRAS G12C mutation who have received at least one prior therapy.
The latest data of Lumakras, which confirmed the long-term clinical effectiveness, is the longest follow-up data among KRAS G12C-mutated NSCLC targeted therapies.
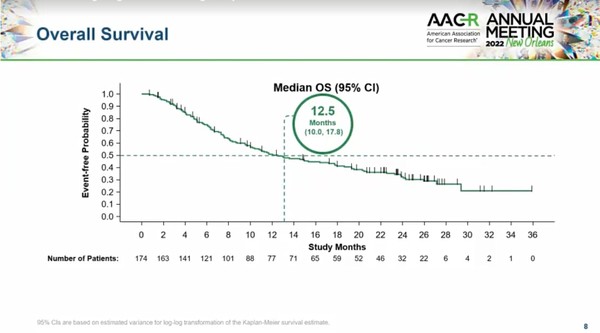
In the CodeBreaK100 study, researchers analyzed the two-year data of 174 NSCLC patients who participated in phase 1 and 2 studies among KRAS G12C-mutated solid cancer patients who previously received chemotherapy or immunotherapy. Among them, 172 had baseline measurable lesions.
The results showed that Lumakras had an objective response rate of 40.7 percent, 12.3 months of median duration of response, and a disease control rate of 83.7 percent. The overall survival was 12.5 months, with 32.5 percent of patients alive at two years. There were no new safety signals in the long-term follow-up.
Kim Soo-a, medical director at Amgen Korea, said Lumacras announced the longest study results for KRAS G12C-mutated NSCLC treatment at the AACR meeting after becoming the first authorized KCRA G12C-mutated NSCLC targeted therapy.
“Amgen is conducting various clinical studies to confirm the therapeutic benefits of Lumakras. We will accumulate evidence for new hope to fundamentally improve the treatment for KRAS G12C-mutated NSCLC patients who could not access targeted therapy,” she said.

