APACMed Digital Health head Roberta Sarno
This month is a momentous occasion, celebrating the nearly 15 years of the formalization of medical device regulations in Korea. Which is also a moment to reflect – how has the industry evolved since then, and what are the major themes to tackle ahead?

APACMed, the voice of the medical device industry in the Asia-Pacific, is going through a similar transformation. While medical devices remain core to our strategy, our members (and the healthcare ecosystem more widely) are demanding a greater focus on the topic of Digital Health. APACMed therefore established a Digital Health Committee in 2020 to provide support along the digital health product journey: from regulatory approval to reimbursement and use.
While regulations for Digital Health are maturing, a gap remains in terms of the value assessment and reimbursement. APACMed published a report on the Digital Health reimbursement topic.
Stakeholders from Korea were key contributors to the report and, hence, we formally launched our Digital Health Reimbursement Alliance (DHRA) in 2022. This article covers the main themes of the discussion and the path forward from here.
“For now, we are covering Digital Health using the current reimbursement system,” said Dr. Kim Joo-youn of the National Evidence-Based Healthcare Collaborating Agency in Korea, contributor to the APACMed report. “In the future, we need to think about new reimbursement categories, and to align the appropriate value of Digital Health with the industry”.
Takeaway #1: Digital Health value assessment and reimbursement strategies must evolve
A first discussion point in Korea is about comparing against the various Digital Health evaluation and financing models around the region and beyond, to align on reimbursement strategy. For example, stakeholders recognize the tremendous efforts undertaken by Korea, along with the UK, France, and Germany, in standing up Health Technology Assessment (HTA) guidelines for Digital Health. Germany has taken a step further, through its Digital Healthcare Act. Many countries see the potential in Digital Health serving as improved connectivity across the care pathways.
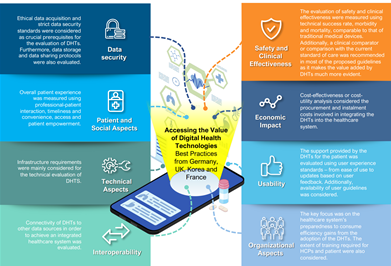
In Korea, stakeholders state that, today, Digital Health is still lumped with other medical technologies like AI-based imaging, pathology, and 3D printing. A suggestion has been made to review the Korea approach to determine, in an evidence-based way, the need to tailor the evaluation and financing flows more specifically to Digital Health.
“The level of attention on Digital Health has increased due to COVID-19, but is still narrowed to only certain types of technologies,” said Suh Jae-hyun of KRPIA.
Takeaway #2: The measurable value of Digital Health in the eye of the payer system
Stakeholders recognize the many initiatives underway in Korea, and the acceleration happening as a result of the COVID-19 pandemic. Now, the key is to be able to measure the progress achieved, and to capture the benefits being provided in areas such as patient experience, care pathway efficiencies, and even healthcare workforce upskilling. Similar to takeaway #1 as well, stakeholders desire to see the scope of Digital Health widened out in Korea.
“We changed the terminology being used from ‘telemedicine’ to ‘Digital Health’ now in order to draw more attention by the Korean government,” said Professor Ahn Jeong-hoon , ISPOR leader and part of the research team at the Department of Health Convergence at Ewha Womans University. “More work is still needed in addressing the Digital Health expenditure and convenience factors though, particularly for patients”.
The story is being heard in Korea. For example, telehealth was previously forbidden, until the COVID-19 pandemic during which temporary permissions have been granted. That said, penetration levels are still <1%, which is considered very low by international standards. Compensation models can be tied accordingly, including with healthcare professionals who could be incentivized to use tools to improve the performance of the health system.
Takeaway #3: Industry perspectives about the status of Digital Health reimbursement
The purpose of the APACMed report is to foster dialogue between governments/payers and industry leaders. For example, by integrating Digital Health into national plans, to form multidisciplinary taskforces, and to design a roadmap for actionable implementation.
In Korea, stakeholders have set the objective to increasingly insert the idea of Digital Health into the evaluation process. In 2022 for example, approval for an electrocardiogram, using a wearable device tester which allows for continuous monitoring up to two weeks, has been achieved. While access to Digital Health is being improved in Korea through such approvals, compensation of fair value remains the open question.
According to the stakeholders, the Korean government is aware of the issue and an official taskforce has been organized to raise the dialogue at the national level. Very much in line with the APACMed report recommendations.
Clearly, there is tremendous opportunity for Digital Health technologies in Korea and the wider Asia Pacific. And yet, without progressive dialogue, we may never hit the ambitious targets. Fortunately, under the auspices of the APACMed DHRA, there is now a platform such dialogue. Further sharing sessions and workshops are being planned in 2022, including our Digital Health Symposium taking place in June (link here). Please join the conversation!

