CHICAGO, Ill. – Pfizer’s first CDK4/6 inhibitor Ibrance (palbociclib), failed to extend overall survival (OS) in the first breast cancer treatment in the phase 3 PALOMA-2 study.
However, the PALOMA-2 study was not designed to check OS data, and it did not include some survival data of patients during the follow-up. Thus, it is not appropriate to conclude that Ibrance failed to prove its effect on survival improvement with these results only, experts said.
At the annual meeting of the American Society of Clinical Oncology (ASCO 2022) on Saturday, Pfizer released the OS data from PALOMA-2, which evaluated Ibrance combined with letrozole in the primary therapy for patients with hormone receptor-positive advanced breast cancer.
The combo of a CDK4/6 inhibitor and endocrine therapy is the standard of care to treat hormone receptor-positive breast cancer in an advanced stage.
Pfizer’s announcement drew more attention because Novartis’ Kisqali (ribociclib), which has the same mechanism as Ibrance, proved OS improvement in the first-line treatment.
Earlier, Kisqali in combo with letrozole reduced the risk of death by 24 percent compared to letrozole alone in the phase 3 MONALEESA-2 trial.
The PALOMA-2 trial compared Ibrance plus letrozole with placebo plus letrozole.
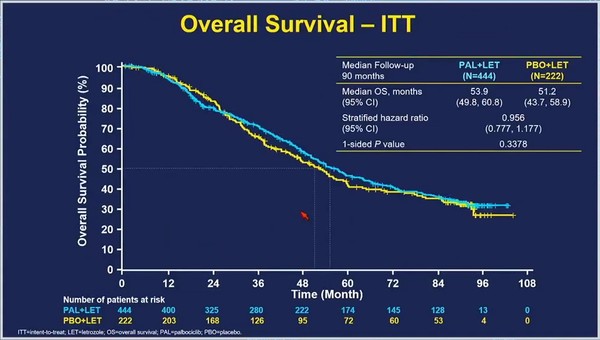
However, after 7.5 years of the follow-up, the Ibrance-letrozole combo group’s median OS was 53.9 months, not significantly different from 51.2 months of the placebo-letrozole group (HR 0.956).
This means that Ibrance plus letrozole combo failed to demonstrate OS benefit compared with letrozole alone.
Richard S. Finn, a professor at the Geffen School of Medicine at UCLA who gave the presentation, emphasized that the PALOMA-2 study included a significant proportion of patients who had less than 12 months of disease-free interval (DFI) with poor prognosis (22 percent in both groups).
He also noted missed survival data from large proportions of patients (13 percent in the Ibrance combo group, 21 percent in the placebo combo group) during the follow-up.
The OS data excluding patients who missed the survival data showed that the median OS of the Ibrance combo group was 51.6 months, slightly longer than 44.6 months of the placebo group. (HR 0.869).
“The analysis reconfirmed Ibrance’s benefit in treatment duration, delaying the time to chemotherapy, and consistent safety profiles, as demonstrated in previous studies,” Finn said.
Still, Ibrance could prove OS benefit neither in the previous PALOMA-3 study nor in the latest PALOMA-2 study. Therefore, it cannot avoid comparison with other CDK4/6 inhibitors anymore.
Professor Park Yeon-hee of hemato-oncology at Samsung Medical Center, who also listened to the PALOMA-2 study results, said it was not right to conclude that Ibrance failed to improve OS with the latest results.
Ibrance is the first CDK4/6 inhibitor, and the PLOMA-2 study did not aim to earn OS data, unlike studies of other CDK4/6 inhibitors, she noted.
Obtaining OS data requires astronomical costs and efforts in a long-term follow-up.
As PALOMA-2 did not aim to get OS data, there were some missed patient data during the follow-up after reaching the primary endpoint – improving progression-free survival (PFS), Park explained.
Thus, it is difficult to judge whether Ibrance improved OS out of these outcomes with missed data.
Moreover, she emphasized that it would be inappropriate to compare Ibrance with Kisqali, which showed OS benefits in the recent MONALEESA-2 trial.
“Unlike PALOMA-2, MONALEESA-2 and MONALEESA-7 of Kisqali, developed later, put enormous efforts to verify OS data from the study planning stage,” Park said. “As mentioned in the presentation, clinical studies of these two drugs have different patient characteristics but the purpose of OS data report. So, it is not right to compare the two.”
While PALOMA-2 of Ibrance included 22 percent of patients with less than 12 months of DFI with poor prognosis, MONALEESA studies of Kisqali excluded patients with less than 12 months of DFI.

