CHICAGO, Ill. – Lilly’s VEGRF2 inhibitor Cyramza (ramucirumab) in combination with Keytruda (pembrolizumab) proved a survival benefit, compared with the existing chemotherapy-based standard of care, in patients with non-small cell lung cancer (NSCLC) whose disease progressed after the first-line immunotherapy.
Dr. Karen L. Reckamp at Cedars-Sinai Medical Center gave an oral presentation on overall survival (OS) data from the phase 2 S1800A trial at the annual meeting of the American Society of Clinical Oncology (ASCO 2022) on Friday.

With immune checkpoint inhibitors (ICI) becoming the first-line standard care for most NSCLC patients who do not have targeted mutations, meeting the needs of patients who progressed after ICI therapy is the key issue.
S1800A drew attention because it was the first study to prove a survival benefit in the second-line setting without a chemotherapy backbone to treat NSCLC patients who developed resistance after using ICI in the first-line treatment.
The research team randomly assigned 130 patients with advanced NSCLC treated with PD-1 or PD-L1 inhibitors to two groups – pembrolizumab plus ramucirumab group and docetaxel plus ramucirumab group. Then, they evaluated OS benefits.
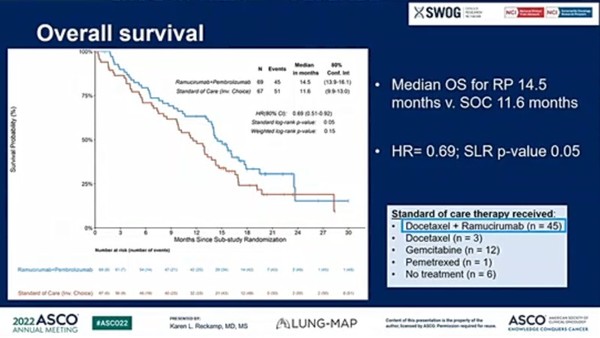
The results showed that the OS of the pembrolizumab plus ramucirumab arm was 14.5 months, reducing the mortality rate by 31 percent compared to the 11.6 months of the docetaxel plus ramucirumab group.
The OS benefit did not depend on PD-L1 expression or the patient’s performance.
However, the benefit of pembrolizumab plus ramucirumab was not observed in progression-free survival (PFS) or objective response rate (ORR), set as secondary endpoints.
Reckamp said it was rare to set OS as the primary outcome in such study settings.
“Using immunotherapy, we observed discordance between ORR/PFS and OS,” she said. “When we planned this study, we worked with a biostatistician to select OS as the primary endpoint, which is even more important as we saw an advantage of ramucirumab and pembrolizumab in OS rather than PFS.”
She emphasized that the study design was different from those of other studies.
“In this treatment setting, many studies use single chemotherapy as a control group. But we used ramucirumab plus docetaxel, which is better than monotherapy,” Reckamp said.
Thus, the researchers not just earned positive results but achieved positive outcomes compared to the optimal standard of care, which is more meaningful, she added.
Citing safety profiles of pembrolizumab plus ramucirumab, Reckamp said the research team had presented treatment toxicities at ASCO 2021.
“After a one-year follow-up, we found that pembrolizumab plus ramucirumab had fewer Grade 3-5 adverse events and more excellent tolerance than chemotherapy,” she said.

