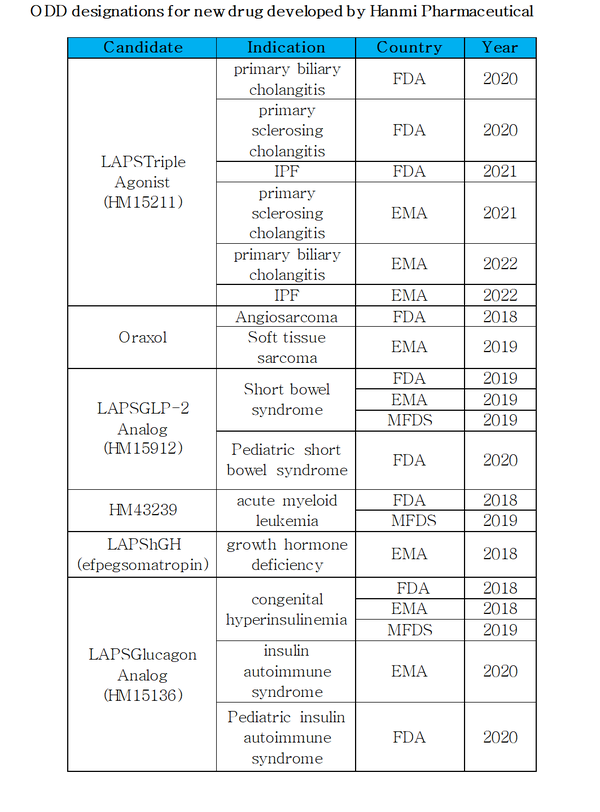
Hanmi Pharmaceutical said Wednesday that the number of its drugs under development that have won orphan drug designations (ODD) has increased to 20, breaking its own record in the Korean biopharmaceutical industry.

Most recently, Hanmi received an ODD from the European Medicines Agency (EMA) for LAPSTriple Agonist (HM15211), its triple action biologic drug for treating idiopathic pulmonary fibrosis (IPF), on Thursday, according to the company.
IPF is a rare disease that can lead to death due to a rapid decline in lung function due to tissue fibrosis resulting from an unknown pulmonary inflammatory process and fibroblast hyperproliferation. It occurs in fewer than 100 cases per 100,000 people every year, but approved treatments lack efficacy, making treatment very difficult.
LAPSTriple Agonist is a triple agonist that simultaneously targets glucagon that suppresses fibrosis, GLP-1 that helps insulin secretion and appetite suppression, and GIP that has insulin secretion and anti-inflammatory action.
Hanmi has confirmed the anti-inflammatory and anti-fibrotic effects of LAPSTriple Agonist in an animal model of idiopathic pulmonary fibrosis.
With the latest ODD designation of HM15211, Hanmi has won 20 designations – nine from the U.S. Food and Drug Administration, eight from the EMA, and three from the Ministry of Food and Drug Safety – for 10 indications in six pipelines.
LAPSTriple Agonist received six orphan drug designations from the FDA and EMA, respectively, for treating primary biliary cholangitis, primary sclerosing cholangitis, and IPF.
“All indications that the company has received ODD cause the fibrosis of specific tissues and have high medical unmet needs,” a company official said. “It is meaningful that advanced regulatory agencies pay attention to the innovativeness of LAPSTriple Agonist.”
Hanmi Pharmaceutical CEO Kwon Se-chang said, “The triple-action biologic drug LAPSTriple Agonist continues to secure significant potential for nonalcoholic steatohepatitis (NASH), the main indication, but also for various rare diseases that cause fibrosis.”
Kwon added that the company would do its best to develop and commercialize LAPSTriple Agonist to improve patients' quality of life suffering from rare diseases.
The ODD is a system that supports the smooth development and approval of treatments for rare, intractable, or life-threatening diseases. Once designated as an orphan drug, the drug developers can benefit from reduced the period required for approval and clinical trials, tax credit, and exclusivity after obtaining authorization.