Effepharm said it has published the results of Uthever® Nicotinamide Mononucleotide (NMN) research on Frontier in Aging, an authoritative international journal on aging mechanisms.
Uthever® NMN, an endogenous compound, has proved safe and improved the NAD+/NADH in the human body, thus realizing the anti-aging function.
The company said its newest NMN clinical trial report offers new insights and more experiences in the field of longevity. The human clinical trial started in 2020 after Effepharm, one of the leading NMN manufacturers, completed animal tests proving that NMN had no acute toxicity.
Frontiers in Aging, one of the most cited medical and pharmaceutical publications worldwide, led the process peer-reviewed by editorial boards of more than 100,000 top researchers. It then agreed to publish Uthever® NMN's human clinical trials, reviewed by scientists, including professors at Harvard Medical School.
It is the first time Effepharm has released detailed trial data to the public.
The main purpose of this trial was to investigate the safety and efficacy of Uthever®, the first branded NMN ingredient.
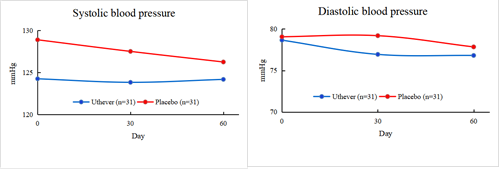
Regarding safety, there was no clinically meaningful derangement in safety laboratory tests and no obvious side effects. Therefore, Uthever®'s parameter for safety evaluation of the anti-aging effect was reduction towards normalization of pulse pressure and systolic and diastolic blood pressure.
On day 30, mean pulse pressures showed a fall of 2.8 percent among the Uthever® group and 5.6 percent among the placebo group from baseline.
Also, the mean systolic blood pressure showed a fall of 0.3 percent among the Uthever® group and 1.1 percent among the placebo group from baseline, and the mean diastolic blood pressure showed a fall of 2.2 percent among the Uthever® group and a rise of 0.2 percent among the placebo group from baseline.
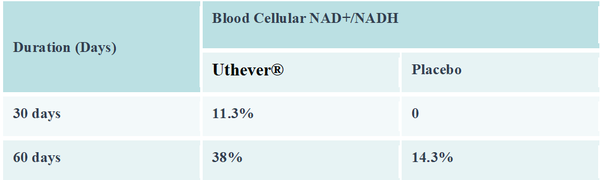
For the treatment's efficacy, the Uthever® group showed a trend of improvement in the cellular blood NAD+/NADH, six-minute walking endurance test, SF-36, and HOMA IR index compared with the placebo group.
As an endogenous compound, NMN cannot be used as a drug to rejuvenate the human body in a short time. Still, the experiment has fully proved the improvement trend of NMN, demonstrating the Uthever® NMN's efficacy.
The primary efficacy parameter, NAD+/NADH levels in the serum, had increased by 11.3 percent in the Uthever® group at day 30, whereas no change was observed in the placebo group.
At day 60, the end of the study, the NAD+/NADH levels were increased further by 38 percent from baseline in the Uthever® group, compared to a mere 14.3 percent rise in the placebo group. The increase in the placebo group may be attributed to the placebo effect in this study.
The results indicate that Uthever® also increases the NAD+ levels in the serum after two months of duration.
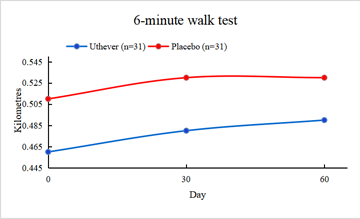
Also, the walking endurance increased by 4.3 percent in the Uthever® group and 3.9 percent in the placebo group on day 30 of the treatment. So no effective difference was seen on day 30 of the treatment for walking endurance.
When the same treatment was continued up to day 60, the Uthever® group showed a rise of 6.5 percent, whereas it remained the same for the placebo group. However, the findings were not found to be statistically significant.
From this analysis, the company said it was clear that the placebo effect was evident until day 30. Still, after that, the Uthever® group showed further improvement in walking endurance, whereas the placebo group remained at the same level.
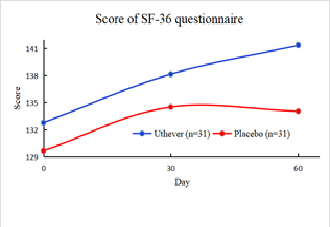
The SF 36 questionnaire, which demonstrates the wellbeing of the subject with a higher score meaning that the person is healthier, showed no significant, meaningful difference between the Uthever® and placebo group at day 30, with the Uthever® group having a 4 percent and the placebo group having a 3.7 percent at day 30
However, on day 60, the Uthever® group showed a rise of 6.5 percent, whereas the placebo group was merely raised by 3.4 percent, showing an almost double increase in the Uthever® group.
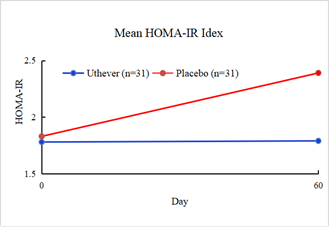
The research also assessed the HOMA IR index, fasting blood sugar, and serum insulin to evaluate the exploratory anti-aging effect on insulin regulation towards normalization.
At the end of the study, the mean HOMA IR index showed a rise of 0.6 percent among the Uthever® group and 30.6 percent among the placebo group from baseline.
Mean Glucose fasting showed a fall of 4 percent among the Uthever® group and a rise of 6.5 percent among the placebo group from baseline.
Mean Serum Insulin fasting showed a fall of 1.9 percent among the Uthever® group, whereas a rise of 26.2 percent among the placebo group from baseline.
The difference in HOMA IR Index between the Uthever® and the placebo groups was not statistically significant.
"The clinical trials of Uthever® NMN is to establish a more professional and scientific image, helping the downstream supplement brand side get more power to do product endorsement and give more confidence to end consumers," Effepharm's R&D Director Jianjun Yu said.

Up to now, more and more well-known supplement brands such as Prohealth Longevity in the USA, Do Not Age in the U.K., Vitanad+ in Japan, AFEGE Anti-aging Shop, and Kenay in Europe are using the Uthever® trademark and realized the co-branding effects, he added.
Yu stressed that Effepharm creatively launched a branded NMN ingredient to bring the purest and safest NMN ingredient around the world.
UTHEVER® NMN, an endogenous compound, has been clinically proven to be safe and to improve the NAD+/NADH in the human body, thus realizing the anti-aging function.
"The first period of NMN clinical trials mainly aimed to verify the safety of UTHEVER® NMN, so we designed the trial with a lower dosage to some extent," Yu said. "In the next few years, we will consider doing more NMN function research to explore the safety of larger doses of NMN and the effectiveness of related indications based on the data from this trial data."
Effepharm will apply for U.S. Food and Drug Administration Affirmed GRAS on NMN, Yu added.


