A group of researchers from Seoul National University (SNU) and the U.S. Stanford University has succeeded in restoring muscle movements in paraplegic rats.
According to the SNU, the joint research team presented the possibility of treating damaged nerves using organic artificial nerves.
A nerve is an organ that regulates and regulates bodily activities by transmitting bioelectrical and chemical signals from the body. However, damage to the nerves can inhibit prompt transmission of biological signals, resulting in permanent loss of some or all of the body's functions, the university said on Sunday.
Therefore, the repair of nerves damaged or degenerated by causes, such as spinal cord injury, Lou Gehrig's disease, Parkinson's disease, and Huntington's disease, has remained an area with a high unmet medical need, it added.
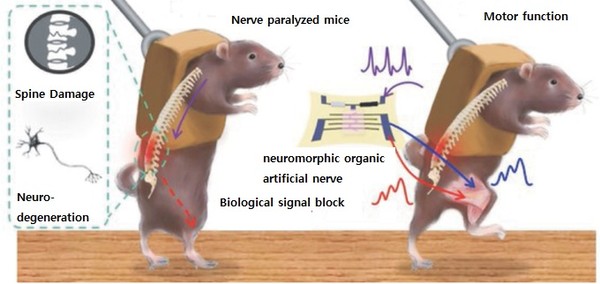
To solve the permanent loss of motor function due to nerve damage., the team -- led by Professors Lee Tae-woo at SNU and Zhenan Bao at Stanford University -- made a neuromorphic organic artificial nerve that mimics human nerves using a sensor that mimics proprioceptors, a sensory organ that detects muscle movement, an organic artificial synapse that mimics the synapse, the junction of neurons, and a stretchable artificial synapse consisting of hydrogel electrodes to transmit signals to the leg muscles.
Afterward, the team inserted the artificial nerve into nerve-paralyzed mice.
As a result, artificial nerves mimicked the structure and function of living nerve fibers to control the movement of the rat leg and muscle contraction. The artificial synapses implemented smooth, natural leg movements without external systems like computers. Also, the mice succeeded in kicking a ball or walking and running on a treadmill.
"Nerve damage remains a challenge despite advances in medical technology. By using engineering technology rather than biological and medical methods, the team has provided a clue that could open a breakthrough for overcoming nerve damage," Professor Lee said. “We expect this approach will open new avenues for people suffering from related diseases and disabilities.”
Lee stressed that the team plans to conduct additional research for clinical applications in humans in the future.
The results of the research were published in Nature Biomedical.

