Boryung said it obtained local marketing approval for finasteride-containing spray treatment for hair loss. Attention is on whether the new product could enlarge its presence in the hair loss treatment market dominated by MSD’s Propecia tablet.
On Tuesday, the Ministry of Food and Drug Safety authorized Boryung’s Finjuve Spray to treat male adults (aged between 18 and 41) with mild or moderate androgenetic alopecia.
Earlier in January, Boryung signed an exclusive agreement with Almirall to sell Finjuve, a spray-type finasteride-based hair loss treatment, in Korea.
The company said it aimed to release the product in the first half of 2023.
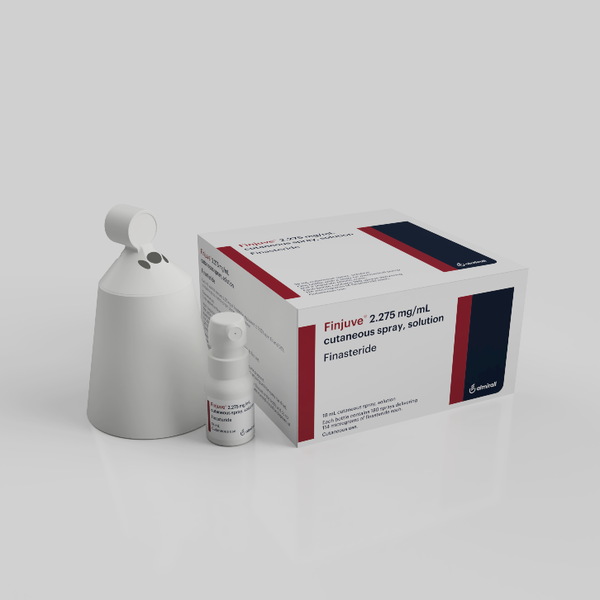
Developed by Almirall, Finjuve is the world’s first spray-type hair loss medicine that uses finasteride. The product obtained approval also in Italy, Germany, Luxemburg, and Portugal.
According to Boryung, Finjuve showed an equal level of efficacy as oral finasteride 1mg in clinical trials overseas. The spray-type treatment’s effect on the human body was much less, as the blood concentration was only 1/100 of that of the finasteride pill.
The spray comes with a cone that can prevent the treatment from spreading to other areas of the scalp.
According to approval conditions, Finjuve Spray should be used in the hair loss area once daily, only on the scalp. Depending on the severity of the hair loss, the user can spray the product one to four times per treatment.
In general, it takes three to six months to see the therapeutic effect, and continuous administration is recommended to maintain the efficacy.
Treatment duration and efficacy should be continuously evaluated. The treatment provides no clinical experience with administration for more than six months.

