The American Society of Clinical Oncology (ASCO) recently revised the guidance for the use of PARP inhibitor (PARPi) therapy in ovarian cancer, saying PARPi monotherapy is no longer recommended to treat recurrent platinum-sensitive epithelial ovarian cancer.
Still, ASCO opened the possibility that PARPi monotherapy may be used in patients with a BRCA mutation, no prior PARPi use, platinum-sensitive disease, and advanced lines of therapy.
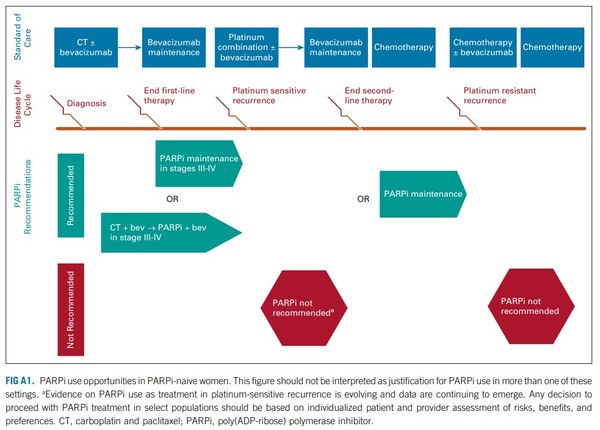
In late September, ASCO published rapid guideline update on PARP inhibitors in ovarian cancer.
The latest revision came after PARPi manufacturers voluntarily canceled indications for recurrent ovarian cancer patients one after another.
In June, Clovis Oncology withdrew Rubraca (rucaparib)’s indication as third-line or higher therapy for ovarian cancer. In August, AstraZeneca nixed Lynparza (Olaparib)’s indication as fourth-line or higher treatment for ovarian cancer.
Both of the two drugs showed a potential adverse trend in overall survival data in the results of recent phase-3 clinical trials that tested them as third-line or higher therapy to treat BRCA-mutated advanced ovarian cancer.
On Sept. 14, GSK also voluntarily withdrew Zejula (niraparib)’s indication of fourth-line or higher therapy.
Accordingly, ASCO renewed the guidance on PARPi use, previously published in 2020.
However, ASCO still recommends PARPi maintenance after platinum-based chemotherapy in newly diagnosed patients and those with first relapse.
Regarding the ASCO’s latest guidance, Professor Kim Jae-weon of Obstetrics and Gynecology at the Seoul National University Hospital said withdrawal of PARP inhibitors’ certain indications in the U.S. should not be exaggerated.
According to Kim, treatment of epithelial ovarian cancer is different depending on the situation of the patient, such as maintenance therapy after primary treatment, maintenance therapy in platinum-sensitive recurrent ovarian cancer, and palliative treatment for those with multiple recurrences and BRCA mutation.
“For this reason, it is necessary to interpret the data according to each situation and apply it to clinical practice,” he said.
The latest ASCO guidance limits PARPi monotherapy only as palliative treatment in those with multiple recurrences and doctors should not suspect PARPi therapies’ effectiveness as maintenance therapy in prior stages, Kim added.
In Korea, Zejula is the only PARP inhibitor that can be used as monotherapy to treat patients with BRCA mutation who have received third-line or higher chemotherapy (regardless of platinum sensitivity) or patients with platinum-sensitive homologous recombination deficiency (HRD) positive recurrent ovarian cancer (including adult patients with fallopian tubes or primary peritoneal cancer).
Regarding the FDA’s withdrawal of PARP inhibitors’ indications, the Ministry of Food and Drug Safety said it was reviewing changing the approval conditions for domestic items.
While the ministry said it would discuss with a pharmaceutical company to take necessary measures, Takeda, the owner of Zejula sales rights in Korea, has yet to decide what to do about it.
“We haven’t made any decision regarding the withdrawal of Zejula’s fourth-line treatment indication in Korea,” an official at Takeda said.
Related articles
- Takeda’s Zejula proves continuous progression-free survival as ovarian cancer therapy
- ‘PARP inhibitors reduce treatment efficacy in relapsed patients of BRCA-mutated ovarian cancer’
- ‘PARP inhibitors increase progression-free survival for ovarian cancer’
- Regulator cuts PARP inhibitor Zejula’s coverage and erases indication

