ATsens, a bio-signal-based healthcare platform company, said on Tuesday that its patch-type long-term continuous electrocardiogram (AT-Patch, ATP-C130) obtained U.S. FDA approval on Oct. 10.
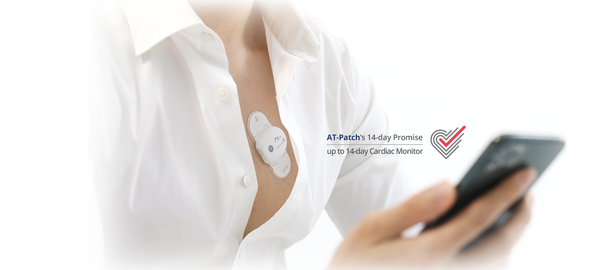
AT-Patch is the first domestic long-term continuous wearable electrocardiogram (ECG) device to be licensed in the U.S. Subsequently, this opens the way for early diagnosis and preventive treatment of heart disease for a continuous 14-day period. Moreover, it is small and light, 8.3mm thick, weighs 13g, and does not require a separate charge or battery replacement.
Furthermore, it includes excellent adhesion with dustproof and waterproof (IP44/IP57) functions, so it can be worn in the shower or during light exercise, minimizing user inconvenience during long-term wear.
The U.S. wearable ECG device market is estimated to be more than 500 billion won (approximately $350 million), the world's largest as of 2021. Currently, iRhythm (product name: Zio Patch), another company that markets wearable ECG devices in the U.S., accounts for the majority of the wearable ECG market.
"AT-Patch was developed focusing on its practical use from the product planning process, and although it is a single channel, it is possible to record clear ECG signals, including P-Waves which are necessary for atrial fibrillation diagnosis,” ATsens CEO Jeong Jon-gook said. "We are pleased to globally verify ATsens in the field of wearable ECGs by obtaining approval in the U.S. following Japan.”
Jeong added that the company will accelerate its entry into the U.S. market as it has successfully passed the largest regulatory gateway.
ATsens has been authorized by Korea’s Ministry of Food and Drugs Safety (MFDS), the European Medicines Agency (EMA), and U.K.’s Medicines and Healthcare products Regulatory Agency (MHRA).

