Wells Bio, a subsidiary of Access Bio, said it has obtained local approval for "careUS COVID/Flu A&B Antigen Duo," a diagnostic kit that simultaneously detects the Covid-19 virus and influenza A/B viruses.
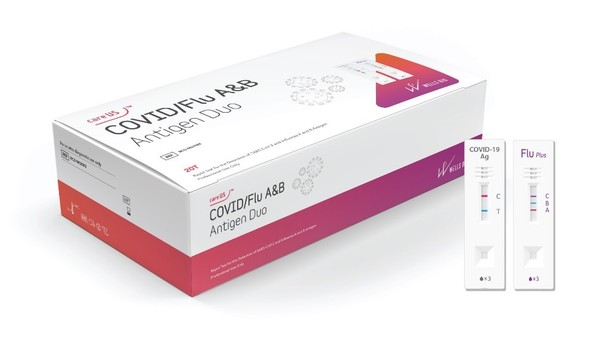
The kit tests SARS-CoV-2 antigen and influenza virus type A and B antigens through an immunochromatographic method using a nasopharyngeal swab for suspected patients with respiratory infection symptoms.
The kit consists of the COVID-19 rapid antigen diagnostic kit (careUS COVID-19 antigen) and the influenza A&B rapid antigen diagnostic kit (careUS Flu A&B Plus), previously approved by the Ministry of Food and Drug Safety (MFDS).
The company said the product is designed to prepare for the possible twindemic -- the dual threat of a severe flu outbreak on top of the Covid-19 pandemic – during the winter season.
According to the company, the latest kit provides test results within 15 minutes, enabling quick reading as well as rapid patient response.
Specifically, the careUS COVID-19 antigen diagnostic kit can detect the major Omicron variants including BA.2, BA.3, BA.4, BA.5, and BA.2.75.
With the approval, Wells Bio plans to start supplying it to local hospitals that want to prepare for the twindemic.
The company also plans to accelerate its advancement into Europe by acquiring Conformité Européenne (CE) certification.
“As the COVID-19 pandemic continues and a flu epidemic advisory has been issued, the sense of crisis in terms of national quarantine is heightening again,” a company official said. “As a company specializing in in-vitro diagnostic medical devices, we will continuously supply diagnostic kits with excellent performance.”

