Pfizer's Ibrance (palbociclib), the first CDK4/6 inhibitor that rewrote the history of treating metastatic hormone receptor-positive/human epidermal growth factor receptor 2 negative (HR+/HER2-) breast cancer, seems to have failed to enter the early treatment market.
Professor Angela DeMichele of the Abramson Cancer Center at the University of Pennsylvania presented the results of the pre-specified cohort analysis of the PALLAS study at the plenary series of the American Society of Clinical Oncology (ASCO) on Tuesday.
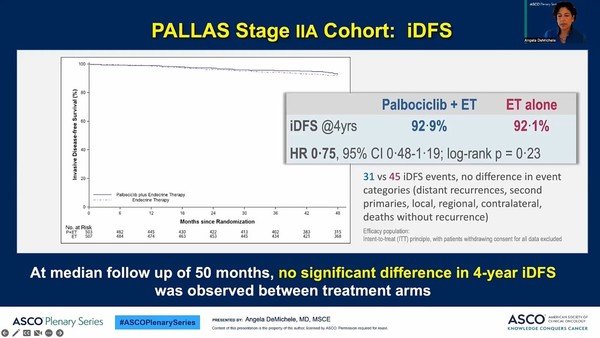
The Pallas study was about the postoperative adjuvant therapy for about 5,760 invasive HR+/HER2- breast cancer patients in the second to third stages, a large-scale clinical trial that compared and evaluated the combination of “endocrine therapy + Ibrance” and “endocrine therapy” alone.
The study failed to win recognition for the usefulness of the additional, paralleled use of Ibrance from the independent Data Monitoring Committee (DMC) in June 2020.
While there were no significant differences between the Ibrance-combined group and the endocrine single therapy group in the invasive disease-free survival (iDFS) as the primary evaluation variable, side effects sharply increased in the Ibrance-added group, forcing the committee to recommend the discontinuation of Irbance administration for participants in the clinical trial.
However, Ibrance's failure was not extended to other CDK4/600 inhibitors. That’s because the MonarchE study of Bergenio (abemaciclip) developed by Lily declared success in the same year the PALLAS study was judged to have failed.
Based on the MnoarchE study results, Bergenio won approval as the combination therapy with endocrine therapy for postoperative adjuvant therapy in early HR+/HER2- breast cancer patients from the U.S. Food and Drug Administration on Oct. 12, 2021.
As the two treatments showed conflicting results in early breast cancer patients, a series of studies analyzing the causes of the PALLAS study’s failure have been announced.
In this process, some cited the differences in underlying characteristics of registered patients in the two clinical trials, and the period of exposure to the treatments was cited as a reason for failure. However, most others agreed that the "drug difference" between the two CDK4/600 inhibitors was the cause of success or failure.
Professor DeMichele looked to see if there were any differences in outcomes according to the stage among early patients through additional analysis based on her premeditated cohort analysis of patients in stage 2A,
According to additional analysis, of the patients enrolled in the PALLAS study, 1,000 were those in stage 2A. In this patient group, the invasive disease-free survival rate in the Ibrance-added group for 50 months during the follow-up monitoring period was 92.9 percent, and that for the single endocrine therapy group was 92.1 percent. In other words, the two groups had no statistically significant difference.
Nor were there significant differences between the two therapies administered on sub-groups differentiating by age, chemotherapy, tumor grade, and clinical risk.
“In the cohort analysis of 2A-stage patients in the PALLAS study, the addition of palbociclib to standard endocrine-based adjuvant therapy has failed to improve results compared to a single endocrine therapy,” Professor DeMichele said. “This shows no benefit of palbociclib in reducing the incidence of early recurrence in early HR+/HER2-cancer patients.
She went on to say, “In the future, we will conduct an integrated analysis of genetic risk and other molecular patterns in a wide range of trans-PALLAS correlation programs. Additional follow-up investigation is underway to assess the effect of palbociclib exposure on the late recurrence of hormonal receptor-positive diseases.”

