The Korea Research Institute of Bioscience and Biotechnology (KRIBB) said on Thursday that they successfully developed a nano-biomaterial that can simultaneously perform precise diagnosis and photothermal therapy (PTT) of cancer cells.

Cancer usually has no symptoms until it progresses considerably, so it is important not to miss the timing of early diagnosis and treatment. Although performing a biopsy after an endoscopy or imaging test is generally used for cancer diagnosis, an easier and simpler diagnostic method is still required.
Similarly, cancer treatment uses surgery, chemotherapy, and radiation therapy, but the treatment is likely to exhibit side effects so there is a high demand for the development of a treatment that maximizes the effectiveness of the treatment and minimizes side effects.
The theragnostic nanocomposite material, MnCO3-mineralized fluorescent polydopamine nanoparticles (MnCO3-FPNPs), was developed using fluorescent materials based on dopamine, a neurotransmitter in the body, and combined manganese salts to have magnetic properties. Accordingly, this enabled the nanoparticle to perform precise diagnosis through fluorescence and magnetic resonance (FL/MR) signals.
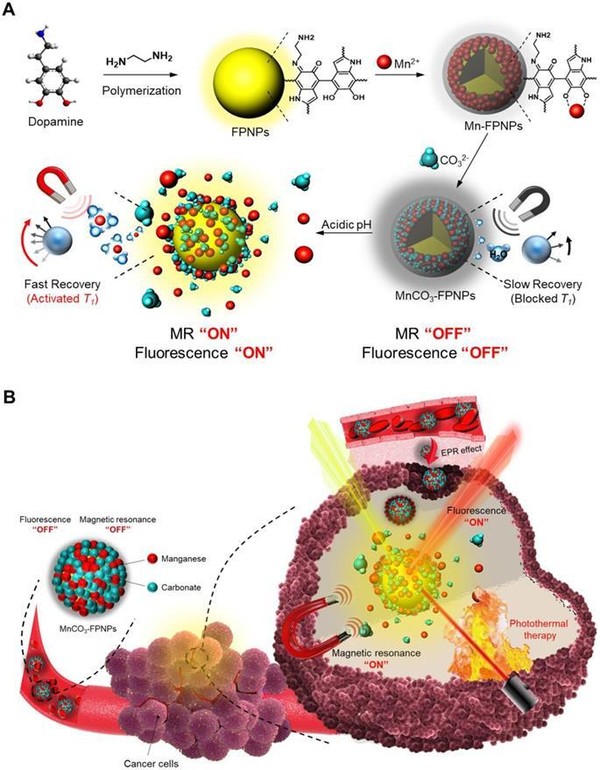
The researchers targeted the acidity near the tumor microenvironment due to the enhance permeability and retention (EPR) effect. At physiological pH, the quenched or “OFF” state of the FPNP fluorescence is maintained. However, at acidic pH in tumor cells, the dissociation of MnCO3 minerals from the FPNPs activates the fluorescence of the FPNPs.
Moreover, under dual FL/MR imaging, the simultaneous near-infrared (NIR) irradiation enables the MnCO3-FPNPs to exhibit photothermal functions by absorbing NIR light and converting it into heat for tumor ablation.
Also, the research team confirmed the suppressed growth and treatment effect of cancer cells in mouse models, increasing its potential application as a nano pharmaceutical material.
"We have developed a material that can drastically reduce the cost and time required for imaging diagnosis and precision treatment by simultaneously diagnosing and treating cancer," Dr. Lee Chang-soo, director of the research said. "We will conduct follow-up studies to target various types of cancer and increase the possibility of human application."
The study entitled “MnCO3-mineralized polydopamine nanoparticles as an activatable theranostic agent for dual-modality imaging-guided photothermal therapy of cancers” was published in the Theranostics journal.

