A group of researchers has recently proved the triple therapy's effectiveness of cytotoxicity-targeted-immuno-cancer drugs in stage-4 HER2 (human epidermal growth factor receptor type-2) benign gastric cancer.

In treating HER2 benign stomach cancer, the combination therapy of cytotoxic anticancer drugs and targeted anticancer drugs is the standard. Some studies have revealed the effects of immuno-cancer drugs alone or the combination of cytotoxic anticancer drugs and immuno-cancer drugs. However, there have been no studies on the triple therapy that adds immuno-cancer drugs to cytotoxic and targeted anticancer drugs.
The joint research team said the response rate of phase-4 HER2 benign gastric cancer patients to the triple therapy had reached 76.7 percent.
Professors Rah Seon-young, Chung Min-kyu, Kim Hyo-song, and Lee Chun-kyu of the Department of Oncology at Yonsei Cancer Center, Professor Emeritus Chung Hyun-cheol of Yonsei University conducted the study in collaboration with Jeolla University Hwasun Hospital, Kangbuk Samsung Hospital, Kangbuk Severance Hospital, and Hallym University Sacred Heart Hospital.
To confirm the triple therapy’s effects on phase 4 HER2 benign stomach cancer patients, the researchers conducted the treatment on 43 patients at five medical institutions, including Yonsei Cancer Center, from 2017 to 2019.
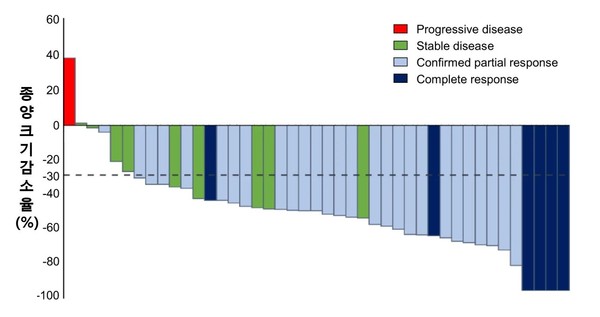
They used capecitabine and cisplatin as cytotoxicity drugs, trastuzumab as a targeted drug, and pembrolizumab as an immuno-cancer drug.
During 18 months, the median value of the follow-up monitoring period, showed good treatment effects with an objective response rate of 76.78 percent. Notably, seven patients could finish their cancer treatment without further progress throughout the entire treatment period of two years.
Progression-free survival (PFS) and overall survival (OS) were 8.6 months and 19.3 months, showing more excellent effects than the existing treatments.
Besides, side effects shown by some patients were related to cytotoxicity drugs, confirming the safety of the further use of immune-cancer drugs.
The research team also pushed ahead with genetic studies of patients’ tumor tissues. As a result of examining patient groups who responded to the triple therapy, researchers found genetic mutations in HER2 gene amplification and RTK·RAS protein signaling pathways that promote cancer growth. In addition, Neoantigen Load, which destroys cancer cells after treatment, increased in these patients.
The team is conducting phase 3 clinical trials. The interim analysis of the phase 3 trials also showed high treatment effects like in phase 2 trials. As a result, the U.S. Food and Drug Administration has given quick conditional approval to the triple therapy under study.
“The study is very significant by revealing the triple anticancer therapy reduces tumors and improves survival in HER2 benign stomach cancer,” Professor Rah said. “Now that the U.S. FDA has granted a quick conditional approval, we expect the triple therapy to emerge as the new standard treatment for HER2 benign gastric cancer.
The study was published in the latest issue of Nature Communications.

