Roche Korea’s Tecentriq (atezolizumab) has expanded its indication to postoperative adjuvant therapy for the first time among lung cancer immunotherapies but fell short of expectations, industry watchers said on Wednesday.
Last Friday, the Ministry of Food and Drug Safety approved the use of Tecentriq as the post-operative adjuvant therapy for stage 2-3A non-small cell lung cancer (NSCLC) patients (according to the seventh edition of UICC/AJCC) with their PD-L1 (programmed death-ligand-1) reaching 50 percent or more of tumor cells (TC) and have undergone resection and platinum-based chemotherapies.
The clinical trial on which the expanded indication was based was the IMpower010 study, which compared the safety and efficacy of Tecentriq adjuvant therapy and BSC (best supportive care) for one year in patients with stage 2-3A NSCLC who have received resection and cisplatin-based platinum chemotherapy.
The primary evaluation variables were stage 2-3A patients with PD-L1 expression rate of 1 percent or more, 2-3A patients in all randomized stages, and disease-free survival (DFS) of 2-3A patients. The secondary evaluation variable was the overall survival (OS) of patients.
According to the first interim result analysis presented at the ASCO 2021 conference, Tecentriq adjuvant therapy proved its efficacy by lowering the disease progression and death risk by 34 percent in 2-3A patients with PD-L1 expression rate of 1 percent or higher. Based on this, the U.S. Food and Drug Administration approved the Tecentriq post-operative adjuvant therapy in October 2021 after a fast-track review.
Tecentriq also showed the same risk-reducing effects on 2-3A patients with a PD-L1 expression rate of 1 percent or higher. It reduced the risk by 57 percent in patients with a PD-L1 expression rate of 50 percent or more and lowered the risk by 13 percent in patients with a PD-L1 expression rate of 1-49 percent. Accordingly, the U.S. FDA also recognized the 13 percent reduction effect in patients with an expression rate of 1-49 percent and finally approved it for patients with a PD-L1 expression rate of 1 percent or more.
However, other countries, including Switzerland, Canada, the U.K., and the EU, approved it only for patients with a PD-L1 expression rate of 50 percent or higher, narrowing its scope of application in what industry watchers saw as a move to focus on patient groups that can experience benefits.
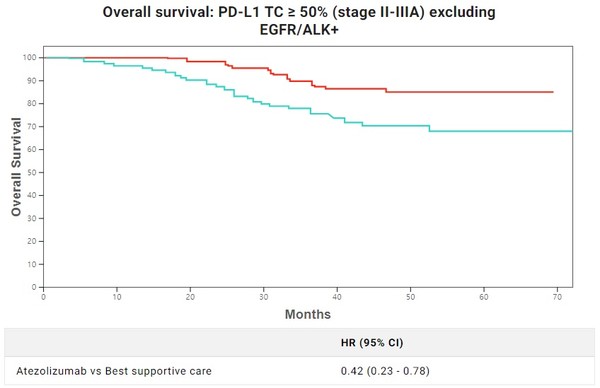
As if to back up such decisions, the new IMpower010 data, unveiled at the WCLC 2022 conference in August, also demonstrated the gains and losses according to the expression rate.
As the follow-up monitoring was prolonged, the patient group with a PD-L1 expression rate of 1 percent or higher showed 29 percent lower risks. In comparison, the patient group with an expression rate of 1-49 percent showed a reduction rate of 5 percent, even lower than the initial 13 percent. In contrast, the patient group with an expression rate of 50 percent and higher showed a similar risk reduction effect despite the prolonged monitoring period, showing a 58 percent of risk reduction rate excluding EGFR (epidermal growth factor receptor) and ALK (anaplastic lymphoma kinase) patients.
The result could be evaluated positively as it showed firm effects in patients with an expression rate of 50 percent or more, not the initial target of patients with a 1 percent or more expression rate. However, the problem was its competitor, Keytruda (pembrolizumab).
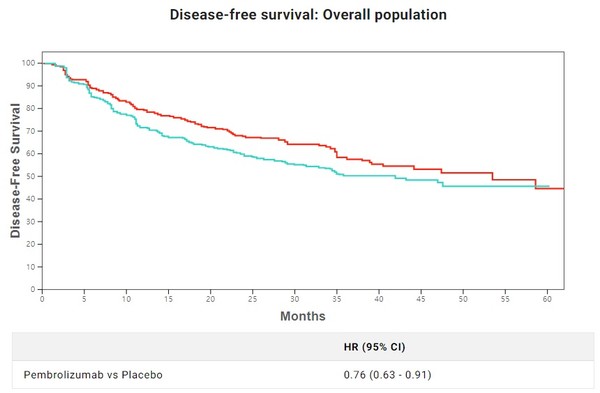
Keytruda is also conducting clinical trials as post-operative adjuvant therapy. The KEYNOTE-091 study targeting 1B-3A stage patients with tumor size of 4 centimeters or more has set all patients’ DFS and DFS of patients with PD-L1 expression rate of 50 percent and higher as its primary evaluation variables and OS of patients as its secondary evaluation variable.
According to the interim result after 18 months, the Keytruda group’s DFS was 73.4 percent compared to the control group’s 64.3 percent. The median value of the DFS was 53.6 months for the Keytruda group and 42 months for the control group, indicating the former reduced death risk by 24 percent. However, in the other evaluation variable of the DFS of patients with a PD-L1 expression rate of 50 percent and higher, the Keytruda groups reduced the death risk by 13 percent but failed to prove any significant improvement.
Based on such results, experts believe that Keytruda has proved it can be used as postoperative adjuvant therapy for 1B-3A stage patients regardless of the PD-L1 expression rate.
Although it is necessary to observe additional monitoring the Keytruda’s clinical trials, Keytruda will likely be used in broader groups of patients than Tecentriq based on the current clinical designs, the observers said.

