The Ministry of Food and Drug Safety has come under fire because of its “poor screening” while discussing providing health insurance benefits for simultaneous test kits to prepare for the “twindemic” of Covid-19 and influenza.
Experts pointed out that the ministry has approved even test kits that can hardly use the term “simultaneous diagnosis.”
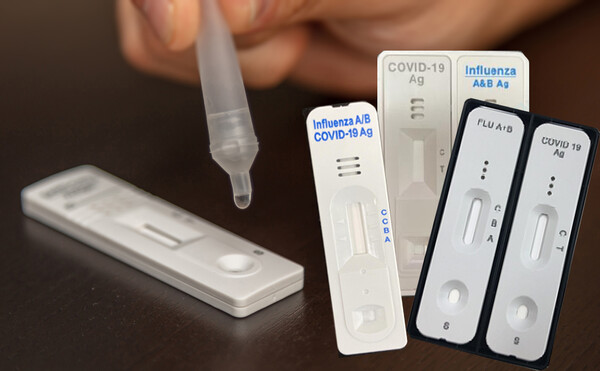
As of November 22, 2022, the ministry approved 21 diagnostic reagents for Covid-19 and influenza. Eleven were PCR test diagnostic reagents, and 10 were antigen test kits for experts.
Causing the controversy were antigen test kits for experts, with eight of the 10 products being just the bundles of Covid-19 test kits and influenza test kits, not fitting for the term “simultaneous diagnosis.” Industry officials call them “duo type.”
Among the 10 duo-type antigen test kits, four collected samples twice and dropped them on devices for Covid-19 and flu separately. “It’s as if you bound a red-color ball-pointed pen with a black-color pen with a rubber band and sold it as a multicolor pen,” a professor of diagnosis and test said.
Other duo-type antigen test kits collected samples once. However, they also must wait after dropping the collected sample on the Covid-19 device and influenza device separately. “They showed a little more sincerity by binding the two ball-pointed pens not with an adhesive instead of a rubber band,” the professor said.
Only two were “combo-type” test kits showing the infection by Covid-19 or influenza with lines on the display after collecting one sample and dropping it on one device.
However, these duo-type products advertise as “one-step simultaneous test kits,” like combo-type products.
“How can the makers call their products simultaneous test kits by bundling a Covid-19 test kit and a flu test kit together? Some of them even must collect samples twice, providing no practical value,” another professor said. “Few doctors would use such products.”
He pointed out that examinees also would seldom accept it if their noses were poked twice for a simultaneous kit, stressing that it was problematic that the regulator approved them as simultaneous kits.
“Isn’t it difficult to call the method of collecting samples separately and dropping them on two separate devices simultaneous diagnosis?” an official at the Korean Society for Laboratory Medicine also said. “By simultaneous diagnosis kits, we usually mean products that collect samples only once and drop them on a device to confirm the infection of Covid-19 or influenza. It’s just common sense.”
Similar criticism was raised during the discussion on whether to provide health insurance benefits to the duo-type antigen test kits.
Last Wednesday, Health Insurance Review and Assessment Service (HIRA) discussed the issue with experts. Most participants reportedly appeared embarrassed faced with the product lists of antigen test kits and product explanations.
Some professors at the meeting said they must object to the ministry, which approved the duo-type products, which were simple combinations of two devices.
“Covid-19 test kits and influenza test kits used for the duo products were products whose safety and efficacy was confirmed through clinical trials at the time of approving individual products,” the ministry said. “We approved those products anew because the makers put individually licensed Covid-129 test kits and flu test kits in a single package.”

