LN Robotics said on Friday that it obtained approval from the Ministry of Food and Drug Safety (MFDS) for its All-purpose Vascular Intervention Assist Robot (AVIAR) in Korea.

AVIAR is a vascular intervention robot with a focus on percutaneous coronary interventions to treat cardiovascular diseases requiring coronary angiography.
The robot first entered exploratory clinical trials in October 2019. After three years of function improvement and document supplementation, it was finally approved by the MFDS.
It features expanded treatment tool operability and unique haptic interface implementation compared to existing overseas competitors' products, said a company official.
Interventional cardiovascular surgery is a procedure to repair blockage or narrowing of the coronary arteries and requires long experience and proficiency on the part of the practitioner due to the variety and complexity of lesions in vascular diseases. The precision control and haptic technology of the robot mimic the movements of experienced practitioners and a multi-channel treatment tool control technology can respond to complex procedures.
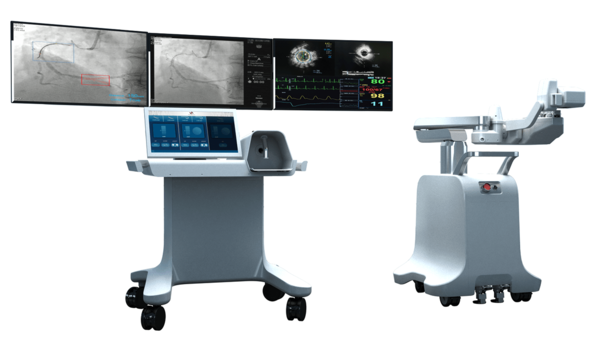
In particular, various clinical applications such as remote intervention for emergency patients and non-face-to-face intervention procedures can be helpful for treating infectious cases and reducing radiation exposure to medical staff.
In 2022, LN Robotics was designated by the Ministry of Health and Welfare to develop medical information visualization and high-fidelity simulation technology related to various cardiovascular procedures.
Using this approval as a stepping stone, LN Robotics plans to continue to develop products with improved control and stability. In addition, it plans to promote a stable commercialization roadmap through clinical demonstrations sequentially through domestic and foreign medical institutions.
“The MFDS' precise product analysis and regulatory guidelines were the biggest reasons for obtaining this product approval," LN Robotics CEO Choi Jae-soon said. "We will accelerate our efforts to build another global leading medical robot company using our own technology."
Professor Kim Young-hak who is leading the clinical development, said, "We will aim to develop more advanced products through various clinical demonstrations in the future.”
Kim added that the approval is an encouraging milestone for Korean cardiologists to be able to use a domestically approved robotic system without relying on overseas technology in robotic procedures.

