Korean researchers said they have developed a real-time reverse-transcription polymerase chain reaction (RT-qPCR) test system that can diagnose the Covid-19 virus with 95 percent accuracy within 10 minutes.
To prevent the rapid spread of highly contagious viruses, it is crucial to detect viruses quickly and accurately.
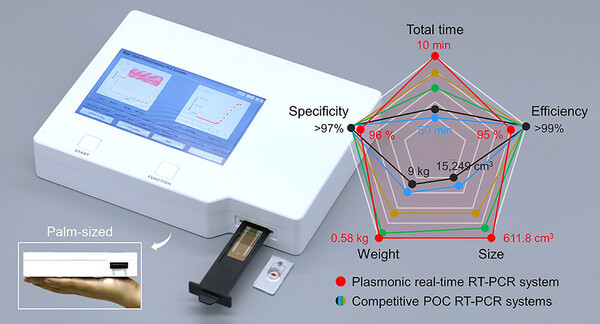
Although RT-qPCR tests are more precise and necessary for definitive confirmation of infectious diseases, they are not suitable for on-site diagnostic tests due to technical constraints.
To address these limitations, a team of researchers at the Korea Advanced Institute of Science and Technology (KAIST), led by Professor Jeong Ki-hun from the Department of Bio and Brain Engineering, collaborated with the National Nanofab Center and Osang Healthcare to develop an ultra-fast and ultra-compact plasmonic nucleic acid analysis system that is well-suited for point-of-care diagnosis.
The team created a handheld plasmonic nucleic acid analysis system for point-of-care diagnosis. The system utilizes micro-nano technology, including an ultrafast plasmonic thermal cycler that uses photothermal nanomaterials, a metal thin film cartridge based on microfluidic lab-on-a-chip technology, and a fluorescence microscope with an ultra-thin microlens array.
The plasmonic thermocycler is fabricated by combining a gold nanoshell structure on a glass nanopillar with a platinum thin film resistive temperature sensor through nano- and microfabrication techniques.
The nanostructure has a very high light absorption rate in the entire visible spectrum, which rapidly converts light from a white light diode (LED) into heat, greatly enhancing the rate of temperature rise.
A thin-film resistance temperature sensor on top measures the surface temperature in real time to realize ultra-fast heat circulation.
The metal thin film cartridge, developed by the team, combines an injection-molded plastic microfluidic chip with an aluminum thin film, which not only increases the reuse rate of expensive nanomaterials but also maximizes cost efficiency.
For real-time quantification in microfluidic chips, the team developed a microlens array fluorescence microscope that utilizes microprocessing technology to simulate an insect’s eyes.
The technology overcomes the limitation of focal length to capture fluorescence images of microfluidic channels at an ultra-close distance of 10 millimeters (mm) and significantly reduces the size of the entire fluorescence system.
To evaluate the performance of the pilot product, the team conducted clinical performance trials and found that the kit successfully distinguishes samples of Covid-19 patients from samples of normal people in a clinical setting with a high accuracy of more than 95 percent.
“The plasmonic nucleic acid analysis system is highly suitable for on-site diagnosis in terms of speed, price, and size, enabling decentralization of diagnostic equipment,” Professor Jeong said. “The team expects that the product will be used for virus detection at quarantine sites such as multi-use facilities and local hospitals.”
The results of the trial were published in the ACS Nano.

