GI Cell said on Tuesday that Drone Treg, a patient-specific regulatory T-cell therapy, was designated as a 2023 Regenerative Medicine-linked Technology Project to be supported by the Korean Fund for Regenerative Medicine (KFRM) in 2023.
GI Cell will receive approximately 17.6 billion won ($13.3 million) in research costs for non-clinical research and phase 1 clinical approval of Drone Treg for four years.
The KFRM will support process development, safety and efficacy evaluation, and GMP technology transfer with the goal of phase 1 clinical approval of candidate therapeutics and therapeutic technologies in the regenerative medicine field.
The KRFM is a funding agency of the Ministry of Health and Welfare and the Ministry of Science and ICT. Its mandate is to provide financial support for research and development projects across the entire cycle of regenerative medicine, from core and source technologies to clinical applications.
The KRFM has been supporting regenerative medicine projects since 2021.
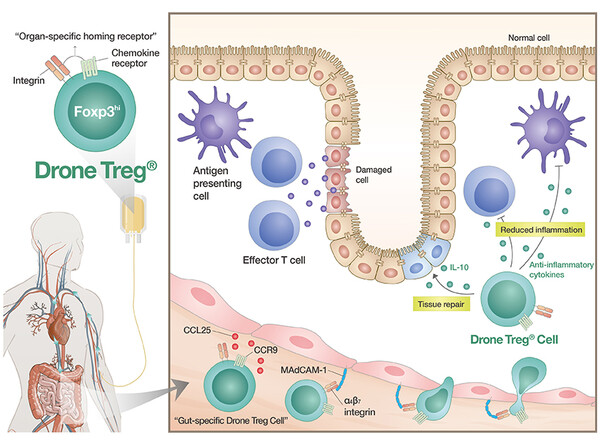
Drone Treg is a patient-specific regulatory T cell therapy that precisely targets specific organs like a drone, with tissue-specific honing receptors precisely expressed in culture. It accomplishes this by controlling the expression of organ-specific homing receptors, Foxp3hi regulatory T cells isolated from the patient’s blood together with gut-specific homing markers, CCR9 and α4β7 integrin.
The company plans to apply its proprietary Immune Cell Pure Expander platform to mass-produce these cells.
Due to investments in regulatory T cells (Treg cells) two years ago, Tregs have recently been in the spotlight with several high-profile deals including a $1.9 billion partnership between GentiBio and BMS for emerging next-generation cell therapy.
"Although the number of patients with autoimmune diseases such as inflammatory bowel disease is steadily increasing, there are still insufficient treatment options for patients who are refractory to existing therapies or have relapsed," said GI Cell’s Chief Scientific Advisor Dr. Jang Myoung-ho. "While there are still many challenges to overcome in the field of Tregs, we are committed to developing innovative cell therapies to provide patients with multiple treatment options."
"This designation recognizes our potential to develop not only NK cell therapies but also Treg cell therapies," said GI Cell’s CEO Dr. Hong Chun-pyo. "We will actively pursue early technology transfer in the non-clinical stage along with the IND approval."

