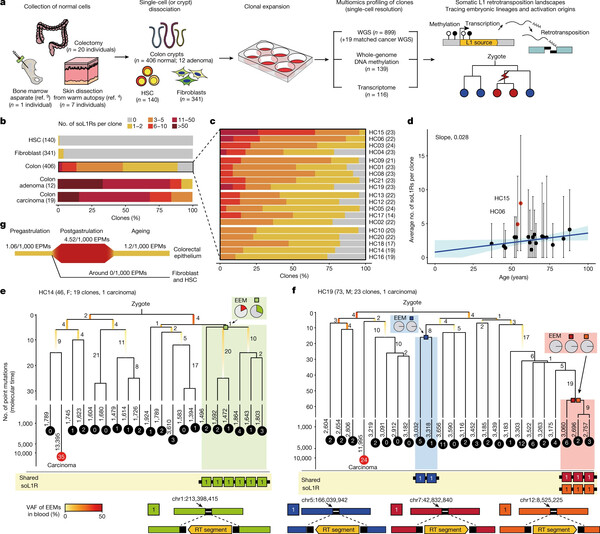
On Monday, the Korean Advanced Institute of Science and Technology (KAIST) announced that its researchers have identified genomic disruptions in human colorectal epithelial cells, confirming that the activation of L1 retrotransposable or "jumping" genes is linked to aging and carcinogenesis.
The activation of L1 jumping genes in the body can cause disruptive innovations in the genomic sequence, contributing to the development of diseases like cancer, but most times, these genes remain inactive.
However, the study revealed that a subset of L1 jumping genes can still be activated in certain tissues to generate frequent genomic mutations during the aging process, which can be linked to cancer development.
Accordingly, the research team led by Professor Ju Young-seok of KAIST’s Graduate School of Medical Science and Engineering used bioinformatics to analyze the whole-genome sequences of 899 single cells from fibroblasts, blood, and colorectal epithelial tissue from 28 individuals.
The frequency of mutations in the L1 jumping gene varied greatly between cell types and was predominantly found in aging colorectal epithelial cells. The team found that genomic mutations in colorectal epithelial cells caused by the activation of the L1 jumping gene occur continuously throughout life, starting in early embryogenesis.
In this regard, when an individual reaches the age of 40, their colorectal epithelial cells have approximately more than one L1 jumping gene mutation, the study found.
Furthermore, in cells with activated L1 jumping genes, epigenetic instability was found mainly during early embryogenesis, confirming that changes in the epigenome are switches that regulate the activity of L1 jumping genes.
The results of this study are expected to help develop methods to control human aging and disease development by identifying the aging and carcinogenic processes caused by L1 jumping genes and inhibiting their activation.
“This study demonstrates that DNA mutations are not the exclusive domain of cells with cancer or disease and that mutations are constantly being generated by the instability of the cells themselves during the aging process of normal human cells,” said Professor Ju.
Professor Kim Min-jung of surgery at Seoul National University College of Medicine also emphasized the need for close collaboration between clinical and basic medicine as human-derived tissues systematically obtained from clinical sites can play a major role in discovering disease processes in humans.
The study was published in the latest issue of the international journal, Nature, on May 10.

