Stage 4 gastric cancer, with a dismal five-year survival rate of under 10 percent, still relies on chemotherapy as the cornerstone of first-line treatment.
Cancer immunotherapies have emerged as a first-line treatment option for HER2-negative advanced gastric cancer, filling a significant void of nearly two decades without a new drug.
They are expected to increase the response rate and duration of response in combination with chemotherapy and ultimately contribute to improved survival rates, experts said.
In Korea, Opdivo (nivolumab), the first immunotherapy to be approved by the Ministry of Food and Drug Safety, is about to win reimbursement two years after its approval. Also, Keytruda (pembrolizumab) has further consolidated its role by improving survival rates through phase 3 clinical trials.
In particular, KEYNOTE-859, a global phase 3 study of Keytruda led by Professor Rha Sun-young at Yonsei Cancer Center, has reaffirmed Korea's status as a gastric cancer treatment powerhouse. The initial data from the study were presented at the ESMO Virtual Plenary session earlier this year, attracting global attention.
At the American Society of Clinical Oncology annual meeting (ASCO 2023) from June 2-6, Rha also presented additional PD-L1 expression data from the KEYNOTE-859 study.
Korea Biomedical Review sat down with Rha on-site on the sidelines of ASCO 2023 to learn more about the highlights of the presentation, how immunotherapies are changing the treatment of stage IV gastric cancer, and their clinical value.

Keytruda improves all endpoints regardless of PD-L1 expression rate
The KEYNOTE-859 study is a phase 3 trial evaluating Keytruda plus chemotherapy (FP or CAPOX) versus placebo plus chemotherapy (FP or CAPOX) in first-line treatment in 1,579 patients with locally advanced or metastatic HER2-negative gastric/gastroesophageal junction cancer.
Initial analysis data showed that Keytruda in combination with chemotherapy provided statistically significant and clinically meaningful improvements in overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and duration of response (DOR) compared to the control group.
The primary endpoint, median OS, was 12.9 months in the Keytruda arm versus 11.5 months in the control arm, with a 22 percent lower risk of death in the Keytruda plus arm (HR 0.78), and the secondary endpoint, median PFS, was 6.9 months versus 5. The risk of disease progression or death was reduced by 24 percent with the addition of Keytruda (HR 0.76), and ORR was 51.3 percent versus 42 percent in the Keytruda arm and control arm, respectively, and median DOR was 8 months versus 5.7 months, respectively.
The data presented at ASCO 2023 was an efficacy analysis based on preset PD-L1 expression rates, which looked at OS, PFS, ORR, and DOR in patients with PD-L1 CPS 1 or higher and CPS 10 or higher.
The results showed that the addition of Keytruda provided statistically significant and clinically meaningful improvements in all endpoints compared to control in patients with PD-L1 CPS 1 or higher and CPS 10 or higher, similar to the overall patient population.
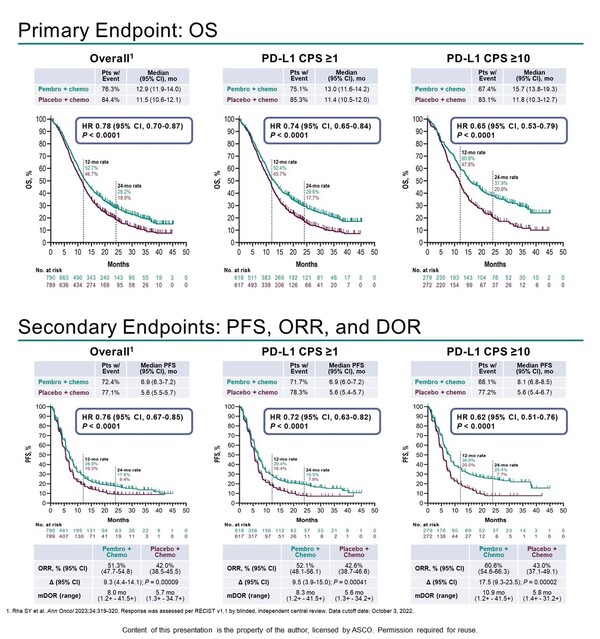
In patients with a PD-L1 CPS of 1 or higher, the median OS was 13 months versus 11.4 months in Keytruda versus control, respectively, with a wider hazard ratio than in the overall population, resulting in a 26percent lower risk of death in Keytruda (HR 0.74).
In patients with a PD-L1 CPS of 10 or higher, the added benefit of Keytruda was even more pronounced, with a median OS of 15.7 months versus 11.8 months in the Keytruda arm and control arm, respectively, and a 35 percent reduction in the risk of death in the Keytruda arm (HR 0.65).
This was also true for the secondary endpoints of PFS, ORR and DOR. In patients with a PD-L1 CPS of 1 or higher, median PFS was 6.9 months versus 5.6 months, respectively, with a 28 percent lower risk of disease progression or death with Keytruda (HR 0.72), and in patients with a PD-L1 CPS of 10 or higher, median PFS was 8.1 months versus 5.6 months, respectively, with a 38 percent lower risk of disease progression or death with Keytruda (HR 0.62).
In patients with a PD-L1 CPS at 1 or higher, the ORR was 52.1 percent versus 42.6 percent in Keytruda versus control, respectively, and the median DOR was 8.3 months versus 5.6 months, respectively. In patients with a PD-L1 CPS of 10 or higher, the ORR was 60.6 percent vs. 43 percent, with a median DOR of 10.9 months vs. 5.8 months, respectively.
The safety profile was consistent with that previously seen with Keytruda, fluoropyrimidine, and platinum-based chemotherapy.
"Based on these findings, Keytruda in combination with chemotherapy may become the new standard of care for the first-line treatment of HER2-negative locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma, regardless of the tumor's PD-L1 expression," said Rha said.
Indication should be 'all-comer' and reimbursement should cover 'PD-L1 CPS 1 or higher'
Rha stressed that for the potential introduction of Keytruda's gastric cancer indication in Korea, future approval should adopt an "all-comer" approach, with reimbursement extended to patients exhibiting "PD-L1 CPS 1 or higher."
Although the KEYNOTE-859 study design showed that Keytruda was effective in all patients, the safety-benefit ratio justifies reimbursement for patients with CPS 1 or higher, she explained.
"However, unlike the CheckMate-649 study, the KEYNOTE-859 study showed a statistically significant benefit in patients with CPS 1-9 with a hazard ratio of 0.83," she said.
"From a physician perspective, I hope that the reimbursement threshold for Keytruda will be set at CPS 1 and above."
The CheckMate-649 study is a licensed clinical trial of the same mechanism of action for Opdivo. In that study, OPDIVO improved OS by 20 percent in combination with chemotherapy compared to chemotherapy alone (HR 0.80) across all patients.

Based on this data, the MFDS expanded Opdivo's gastric cancer indication to all-comers, but reimbursement is currently limited to patients with PD-L1 CPS 5 or higher. In patients with PD-L1 CPS 5 or higher, the OS hazard ratio for Opdivo compared to control was 0.71, further narrowing the reimbursement criteria to patients with CPS 5 or higher.
Furthermore, when researchers recently analyzed patients with CPS 1-4 in the CheckMate-649 study, they found no statistically significant benefit from the addition of Opdivo.
Rha emphasized that CPS 1 is a much clearer criterion than CPS 5 for screening patients in real-world clinical practice.
"CPS 5 can mean different things to different people, but CPS 1 is the difference between having it and not having it," Rha said. "The KEYNOTE-859 study showed a clear benefit of adding Keytruda in patients with CPS 1 or higher, so we can use it with more confidence in the clinic."
Related articles
- Why doctors should use immunotherapy for early lung cancer patients
- Korean cancer researchers present 139 studies at ASCO 2023
- ‘Palliative care neglected amid a flood of expensive cancer drugs’
- [ASCO 2023] Tagrisso shows ‘breakthrough’ benefit in early lung cancer
- [Photo News] ASCO returns to full strength, with Korean firms actively engaged
- Promising 3-drug combo discovered for HER2-positive advanced gastric cancer
- 'Joint reimbursement of Opdivo, CDx platform marks milestone in gastric cancer but challenges remain'

