The global influenza vaccine market is experiencing a significant revival as the Covid-19 pandemic gradually subsides.
The influenza virus, which causes seasonal flu, poses a significant threat each year, resulting in substantial morbidity and mortality globally.
With Covid-19 now relatively under control in many regions, healthcare authorities and professionals are urging individuals to get vaccinated against influenza to minimize the burden on healthcare systems already stretched thin due to the ongoing pandemic.
In Korea, the market is also experiencing a tectonic shift with the return of SK bioscience, which was the leading influenza vaccine supplier until 2021, which is when the company left the market to develop a Covid-19 vaccine.
During that time, GC had acquired most of the flu vaccines ordered by the government, which SK Bioscience used to almost monopolize.
According to industry estimates, GC supplied over 60 percent of the 2.8 million doses of flu vaccines ordered by the government in 2022.
While industry watchers speculated that SK bioscience will have a difficult time regaining the market that it had lost, SK bioscience, which returned to the national procurement market after a two-year hiatus, came on top in this year's National Immunization Program (NIP) procurement market by supplying the largest amount of doses.
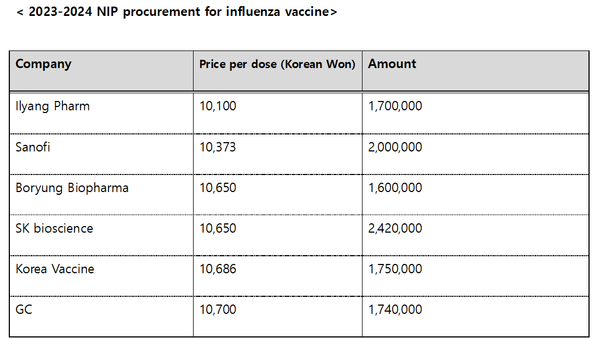
According to the Korea Disease Control and Prevention Agency, SK bioscience will supply 2.42 million influenza doses for the 2023-2024 NIP procurement for influenza, which accounts for a 21.6 percent share of the total 11.21 million doses purchased by the government.
Compared to SK bioscience, GC will supply 1.74 million doses of its vaccine, ranking fourth among the six suppliers.
Regarding the matter, a GC official said in the NIP market, bid price matters, and its competitors offered lower unit prices than what the company expected.
"The government assigns the number of doses based on the bid price and then it's a matter of how many doses that a company can manufacture," a GC official told Korea Biomedical Review.
GC winning influenza procurement markets abroad
However, compared to the domestic situation, GC is leading the global procurement market for influenza vaccines at a time when the market is expected to grow.
According to Evaluate Pharma, a global market research firm, the global influenza vaccine market is expected to grow from $5.8 billion in 2020 to $7.4 billion in 2025.
Notably, GC maintained the largest flu market share in Latin America after winning a $43.38 million contract with Pan American Health Organization (PAHO) to supply its influenza vaccine to Latin America.
The vaccines will be supplied to Latin American countries in the first half of the year.
With the latest bidding win, GC maintained the top market share position for PAHO's southern hemisphere influenza supply for the past decade.
GC's GC Flu Multi Inj. also has product approval in 23 countries, compared to 12 for SK bioscience's SKY Cellflu Inj.
GC is also planning to develop a messenger ribonucleic acid (mRNA-based) flu vaccine in earnest through GC Biopharma, it's subsidiary.
The company plans to develop this mRNA vaccine based on the lipid nanoparticle (LNP) development and option agreement signed with Canada-based bio company, Acuitas Therapeutics, in April last year and a recent non-exclusive licensing agreement involving LNP technologies.
GC Biopharma aims to utilize its technology and experience in developing influenza vaccines together with the proven technology of Acuitas to enter phase 1 clinical trials by 2024.
SK bioscience plans to expand its global market share based on the fact that SKY Cellflu Inj. is the world's first quadrivalent cell culture vaccine to receive WHO's prequalification (PQ) certification.
The company received such a certification in 2020.
Related articles
- [Top K-Pharma Analysis ⑧] GC’s 2022 earnings expected to be largest in company's history
- [Top K-Pharma Analysis ④] SK Bioscience faces trouble as sales, operating profit fall drastically in 2022
- SK bioscience, Australian institute collaborate to bolster global infectious disease ecosystem
- SK bioscience, Sanofi's pneumococcal vaccine shows strong efficacy in p2 study
- SK bioscience acquires 7% stake in cash-strapped Novavax, forging long-term partnership
- SK bioscience says cell-cultured influenza vaccine will become new norm

