Lundbeck is drawing the industry’s attention by saying it would begin a phase 2 clinical trial of an autoimmune disease candidate it licensed in (L/I) from Korean biotech Aprilbio.
Through a conference call for the first half-year on Wednesday (local time), Lundbeck announced it plans to start the phase 2 trial of the candidate, Lu AG22515, in 2024.
Lu AG22515 is an autoimmune disease treatment candidate that Lundbeck acquired from Aprilbio in 2021. Its original development name was APB-A1.
The two companies drew the market’s attention by signing a contract worth $448 million (about 610 billion won), including an upfront payment of $16 million.
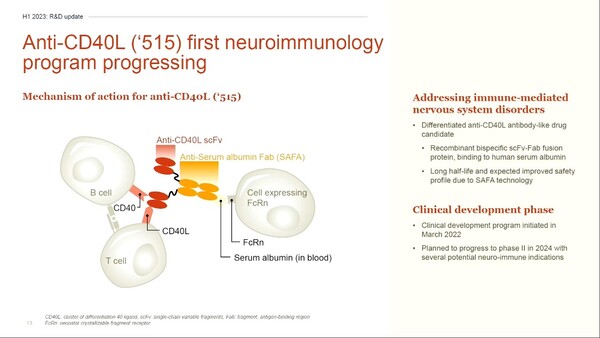
Lu AG22515 is an antibody fusion protein that inhibits immune activation by blocking the interaction and co-stimulatory signaling of the immune checkpoint molecules CD40 and CD40 ligand (CD40L).
Lu AG22515 features a long half-life in the body by binding to CD40L and human serum albumin. It used Aprilbio's long-lasting recombinant protein platform technology, SAFA (serum-albumin bispecific antibody-fragment).
"We are on track to enter phase 2 for proof-of-concept (POC) purposes next year," said Johan Luthman, vice president and head of research and development at Lundbeck. "Phase 1, which began in March of last year, is progressing rapidly across the board, including dose escalation studies.
According to the U.S. National Institutes of Health (NIH) clinical trials site ClinicalTrials, the Lu AG22515 phase 1 study is "active, not recruiting.” The trial has passed its three-day end-of-study deadline this month but is believed to be in its final stages.
In addition, Lundbeck hinted at the possibility of expanding the indications for Lu AG22515 through the phase 2 trial next year.
"We will be exploring several potential immune disease indications, and we are very excited to showcase our differentiated CD40L binder," Luthman said. "We are confident that we are on track to build our mid-stage pipeline and have the potential to move two to three assets into phase 2 during 2024."
As Aprilbio’s global partner formalizes its entry into phase 2, the original developer has come to expect to receive additional milestone payments upon subsequent clinical entry next year, industry watchers said.
In addition, the initiation of Lu AG22515’s phase 2 clinical trial could increase the value of the SAFA platform and lead to further technology transfer, they added.
In Australia, Aprilbio is conducting a phase 1 clinical trial of APB-R3, an inflammatory disease treatment candidate with SAFA platform technology.
In August last year, the company signed a joint research and development agreement with Yuhan Corps. to develop new drugs for intractable solid cancer.

