The Ministry of Food and Drug Safety (MFDS) has designated HeartMedi, AiMEDiC’s cardiovascular risk assessment software that evaluates the degree of stenosis of coronary arteries, and DrNoon CKD, Mediwhale’s hospital treatment software that displays the risk of chronic kidney disease, as the 41st and 42nd innovative medical devices.
AiMEDiC’s HeartMedi is the first product in Korea to evaluate the degree of stenosis of coronary arteries by forming coronary arteries in three dimensions based on chest computed tomography (CT) images and calculating fractional blood flow reserve (FFR) based on computational fluid dynamics.
It was designated as an innovative medical device in recognition of its technological innovation in integrated review and evaluation.
FFR is a ratio (0~1) of the maximum blood flow of normal blood vessels near and far from the coronary artery stenosis, and the lower the ratio, the more severe the intravascular stenosis.
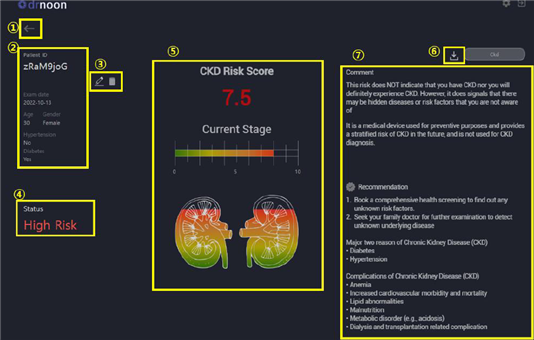
Mediwhale’s DrNoon CKD is also the first product in Korea that analyzes the structure of the retina and the shape of blood vessels in the retina from retinal photographs using artificial intelligence to assess the risk of developing chronic kidney disease as low, moderate, or high risk. The product was designated as an innovative medical device in recognition of its technological innovation in the general review.
Currently, the standard test for chronic kidney disease checks the glomerular filtration rate based on blood tests. However, the latest product predicts the occurrence of kidney disease by analyzing microscopic blood vessel changes in retinal photographs based on research showing that retinal vascular abnormalities and the risk of chronic kidney disease are closely related.
HeartMedi, which has undergone integrated review and evaluation, has applied for a sales license and is being reviewed. Upon completing the item license, it is expected to enter the medical field immediately without reimbursement and be used for three to five years.
DrNoon CKD, which has undergone general review, is a product under development and is likely to be subject to special exemptions for approval review, such as priority review, resulting in rapid commercialization.
“We will continue to operate the innovative medical device system effectively so that innovative medical devices can be widely used in the medical field and quickly provide safe and new treatment technologies to the public," the ministry said.

