The Korea Health Industry Development Institute (KHIDI) said on Monday that a multidisciplinary Korean research team demonstrated the process of fibrosis of tau protein, which has been identified as the pathogenesis of Alzheimer's disease, and the mechanism of formation of neurotoxic substances.
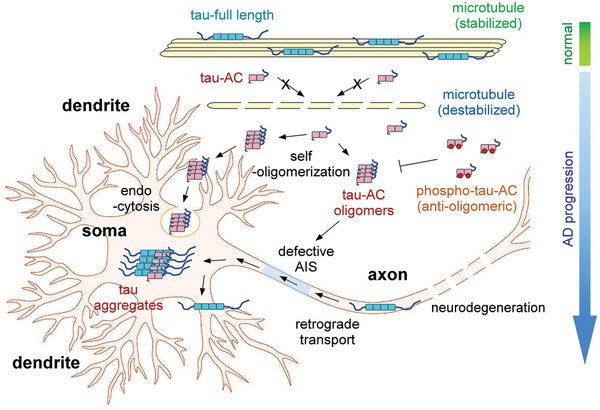
In particular, they identified the fibrosis of tau proteins, including the infiltration of tau protein fragments into brain nerve cells, the inhibition of synaptic function, and the effects on memory loss and brain tissue death in animals.
In Alzheimer's disease, the leading form of dementia, existing research suggests that amyloid beta and tau proteins build up in the brain and form toxic substances that kill nerve cells, but the more fundamental pathogenesis remains unclear, hindering the development of treatments.
The study led by Professor Lee Min-jae of Seoul National University College of Medicine (SNUCM) and Professor Kim Jun-gon of Korea University investigated aggregation cores that promote the formation of neurotoxic substances to elucidate tau protein fibrosis at the molecular level.
They found that a portion of the internally cleaved tau protein can spontaneously form neurotoxins under physiological environmental conditions without any treatment and that it can convert even normal tau protein into neurotoxins. This is because the cleavage of the protein exposes the aggregation core.
Furthermore, the researchers identified the pathways by which the neurotoxins generated by the cleavage of tau enter neurons, induce further aggregation, and reduce synaptic plasticity, thus establishing their pathogenic mechanisms at the cellular level.
In addition, animal model experiments confirmed that injecting tau aggregation cores into the hippocampus in the ventricles of mice caused neuronal death, neuroinflammatory responses, and behavioral changes similar to Alzheimer's disease, such as memory loss. This confirmed that the pathological mechanisms of tau fragments identified at the molecular and cellular levels are reproduced in animal models.
"This study has revealed the principles of fibrosis and neurotoxicity of the new tau protein at the molecular, cellular, and animal model levels and that further research will contribute to suggesting new treatment methods for Alzheimer's disease,” said Professor Lee.
The results of the study were published in the international journal of Advanced Science on Aug.18.

