Ybrain, a company specializing in mental health therapeutics, said on Wednesday that a clinical trial conducted in the U.S. using its depression electroceutical, MINDD STIM, showed efficacy in improving immediate depression symptoms.

Depressed patients have difficulty interpreting other people's emotional expressions, which can lead to high rates of recognition errors. This has been linked to increased activity in the amygdala and decreased activity in the left frontal lobe, which is responsible for processing emotional information, leading to an attentional bias that focuses on negative stimuli.
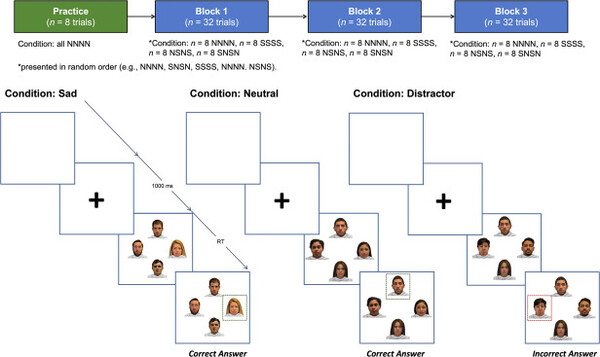
The research team at NYU Langone's Department of Neurology designed a clinical trial to measure how well depressed patients can recognize facial emotions quickly and accurately, and how emotional changes affect their attention using MINDD STIM transcranial direct current stimulation (tDCS).
The trial ran for six months from June to December last year and 20 women with mild to moderate depression were compared to 21 healthy women to determine the speed of cognitive response to immediate facial expression recognition changes before and after tDCS application to the left frontal lobe.
The clinical trial found that a single use of MINDD STIM improved the negative attentional bias of depressed patients, and they also reported feeling less depressed and happier after treatment.
In particular, the participants with depression differed from the control group in emotional processing speed and accuracy in identifying sad stimuli before tDCS treatment. This showed that depressed patients had a concomitant deterioration in attention that allowed them to recognize negative stimuli more quickly but with poorer accuracy.
In response to the tDCS treatment, the participants with depression became significantly faster on the distractor condition, suggesting a specific reduction in bias toward negative emotional information.
Additionally, the depressed group also had significant improvements in self-reported mood including increased happy, decreased sad and anxious mood.
"It is very encouraging to see the immediate improvement in depressive attentional bias of MINDD STIM on the global stage through the U.S. clinical trial," said Ybrain CEO Lee Ki-won. "We expect that the face recognition visual search task utilized in this trial will serve as an excellent indicator of MINDD STIM’s effectiveness in treating depression."
The results of the study were published in the international journal Neuromodulation on Aug. 20.
Related articles
- Ybrain wins ₩2.4 bil. won state funding to develop electroceutical for perinatal depression
- Electroceuticals empower Korean biopharma's growth
- Ybrain provides real-world evidence for developing global tCDS industry standards
- Ybrain partners with Friend & Companion to expand neurological diagnostics market with MINDD SCAN
- Ybrain and Meditrix to collaborate on mind health digital devices

