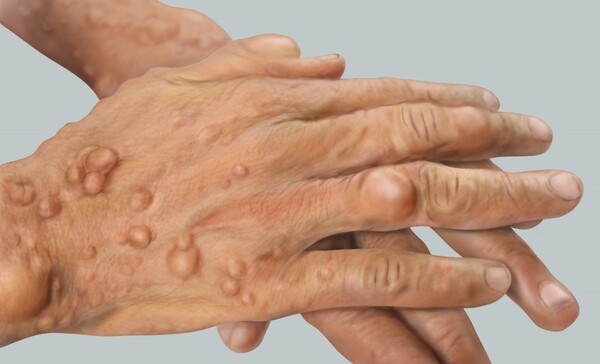
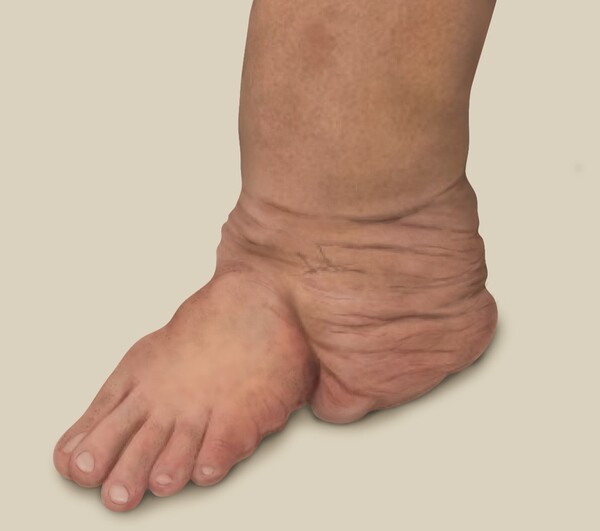
Neurofibromatosis is a rare inherited disease caused by abnormalities in the NF1-NF2 genes, which causes tumors to form in various parts of the body, including the skin of exposed areas, such as the face, neck, hands, and feet. Most tumors caused by neurofibromatosis are benign, but about 2-5 percent progress to malignancy, and the five-year survival rate for malignancy is less than 50 percent, which is lower than the five-year survival rate for all cancers, which stands over 70 percent in Korea.
Even if neurofibromatosis is not a malignant but a benign tumor, its lethality varies, depending on which part of the body it occurs. Typically, neurofibromatosis in the brain, which controls the body, the spinal cord, the central nervous system protected by a strong spine, and the neck, which contains the airway, are among the most deadly neurofibromatosis.
Lim Ji-hoo, 15, diagnosed with neurofibromatosis when he was less than a year old, also had a cluster of benign tumors in a critical area, his neck. At the time of his diagnosis, Lim's father, Soo-hyun, was told by doctors at Samsung Medical Center, Severance Hospital, and Seoul National University Hospital that "a neurofibromatosis on the inside of his right neck could grow as he grows, blocking blood vessels or pressing on nerves, the esophagus, or the airway."
A blood vessel or nerve to the head or an airway to the lungs could be life-threatening. At this point, neurofibromatoses are benign tumors and should be treated to remove them, but those in the brain, spinal nerves, and neck are not easy to remove. The risk of surgical side effects from removing neurofibromatosis in such places outweighs the benefits of treatment. Therefore, all medical professionals said, "We will try surgery when there is a problem with living.”
To Lim, those words sounded like an invitation to die. However, at the time, the doctors advised him that "parents of children with rare diseases should study." So he could find another way. Desperate to save his son's life, he scoured Korean and international medical magazines and found a case in Cell, one of the world's top three medical journals, which treated a child with neurofibromatosis exactly like junior Lim.

At that time, a research team led by Dr. David Wade Clapp at Riley Hospital for Children, an affiliate of Indiana State University in the United States, had successfully treated a girl around Lim's age with neurofibromatosis in her neck with the blood cancer drug Glivec (imatinib), and the results of the treatment were published in Cell.

After reading the paper, he emailed and called Dr. Klapp desperately, asking him to "save his son. But Dr. Klapp did not respond. Dr. Klapp's secretary answered the phone and left a note, but that was all. However, Lim Soo-hyun was too desperate to stop calling for help. Finally, Dr. Klapp gave him a simple answer: Contact Dr. Kent Robertson, another research team member.
Through a series of twists and turns, Lim could get in touch with Dr. Robertson and fully explain her son's condition. The team was conducting a clinical study of Glivec in neurofibromatosis, and Lim pleaded with Dr. Robertson to allow his son to participate in the study. However, participation in the study fell through. That was because the study subjects were narrow to only Americans.
"It was a time when clinical research in the U.S. was expanding widely but contracting back after the global financial crisis in 2009, so foreigners were excluded from the study," Lim said, explaining that her son could receive Glivec in December 2011 after the research team worked with him to find a treatment option. The researchers made an unusual offer; they would cover all of a U.S. hospital's very expensive medical expenses, except for the cost of Glivec.
Not just U.S. doctors but Korean physicians helped Lim with the Glivec treatment. Dr. Lee Su-jeong, a pediatric blood cancer specialist at Samsung Medical Center, Dr. Jung Sang-bong, a neurosurgeon at Samsung Medical Center who is a friend of Lim's, and Dr. Lim Go-woon, a pediatrician and Jung's wife, actively came forward for Lim son's Glivec treatment.
Dr. Lee arranged all the tests and other materials the U.S. researchers wanted, and Drs. Jung and Lim held twice-monthly 7 a.m. "conference calls" with the U.S. medical team to help them prepare for the son’s Glivec treatment. In December 2011, six months after the first conference call, Lim, his wife, and his son boarded a plane to the U.S. The first dose of Glivec was administered to the young Lim at Riley Hospital for Children.
After a month of treatment in the U.S., Dr. Lee continued to monitor the boy’s progress on Glivec, performing tests as required by the research team, while Drs. Jung and Lim continued conference calls with the U.S. medical team. The entire family traveled back and forth to the U.S. three times, six months apart, to keep up with their son’s treatment. With the full cooperation of the Korean and U.S. medical teams, the junior Lim took Glivec for about 20 months.
However, the results were not as dramatic as those reported in Cell.
"The neurofibromatosis didn't shrink significantly in size. It was ambiguous, but the doctors said, 'it did not decrease much, but it decreased very slightly'," Lim recalled. As Glivec did not work as expected and several other issues occurred, the boy’s Glivec treatment was eventually discontinued.
However, it was far from abandonment. When Lim Soo-hyun Lim learned about the availability of a new drug for neurofibromatosis through the Children's Tumor Foundation, a private organization dedicated to genetic diseases affected by the NF gene, such as neurofibromatosis, he turned to a new treatment. That new treatment was AstraZeneca's neurofibromatosis drug Koselugo (selumetinib).
When Dr. Lee Su-Jeong, who had been so helpful with the boy’s treatment, left Samsung Medical Center, she introduced him to Dr. Lee Beom-hee, a pediatrician at Asan Medical Center, who was working on neurofibromatosis. Dr. Lee knocked on AstraZeneca's door and offered to conduct a clinical study of Koselugo in Korea, which soon became a reality.
It wasn't easy for Lim to take a month off every six months for his son's treatment, and the cost of traveling back and forth to the U.S. was a factor that couldn't be ignored. Lim could discontinue her son's Glivec treatment because he knows his son would do well until Professor Lee conducts Koselugo’s clinical trial in Korea.
The family had been waiting for Professor Lee's Koselugo clinical study in Korea, which began in 2019. Their son was selected as one of the first 10 patients and began treatment with Koselugo in October of that year. Unlike Glivec, the effects of Koselugo treatment came quickly for the young Lim.
"My son had to wear a positive airway pressure machine every night because his apnea was severe, and he was not good enough to sit up all night if he had a cold during the season. However, within a few months of taking Koselugo, he was good enough to return the machine," the senior Lim said. Today, his son is still being treated with Koselugo, thanks to the clinical study, and he no longer has to worry about the potentially fatal consequences of a benign tumor in his neck compressing blood vessels or nerves to his brain or airway.
However, aside from Professor Lee's clinical study participants, other patients with neurofibromatosis in desperate need of treatment remain untreated.
Meanwhile, Koselugo has been officially licensed in Korea. Classified as a "treatment for life-threatening or serious diseases for which there are no existing therapies," Koselugo was designated the first drug subject to expedited review in Korea in October 2020 and was officially licensed in May 2021. However, its reimbursement process was completed over two years ago.
On Sept. 7, Koselugo crossed the Health Insurance Review and Assessment Service threshold, the first gateway to benefits. However, it must still pass the Health Insurance Policy Review Committee under the Ministry of Health and Welfare and negotiate the drug price with the National Health Insurance Service.
Lim, who understands the situation of neurofibromatosis patients and their families who desperately want the drug to be covered, feels sorry for the current situation where Koselugo is not covered and cannot be used.
That's why, in early September, he and the Korean Federation of Rare and Incurable Diseases filed a complaint to the National Human Rights Commission, calling for Koselugo to be covered as soon as possible.
"I know how they feel, and I don't feel comfortable because I've been there," Lin said, emphasizing the need to reimburse the drug. “Koselugo should be reimbursed as soon as possible so that other patients with neurofibromatosis in desperate need of treatment can also administer it."

