With a dream to revolutionize the field of diagnostics, Eone Diagnomics Genome Center (EDGC) is charting a way forward to use methylation patterns as a way to encourage early disease detection, starting with cancer and hoping to expand to neurodegenerative diseases such as Alzheimer's disease and infectious diseases such as Covid-19.

“The beauty of our technology is that it does not matter if it is lung cancer, colon cancer, breast cancer, Alzheimer's disease or any other disease, we will still extract the same 65,000 hypomethylation signals from the blood to recognize typical patterns and diagnose disease at an early stage,” said EDGC CEO Lee Min-Seob.
EDGC is an international joint venture established in 2013 between EONE Laboratories in South Korea and Diagnomics in San Diego which has developed leading-edge technology for genome analysis. The CEO highlighted his experience at Sequenom with cell-free DNA (cf-DNA) analysis for non-invasive prenatal (NIP) diagnostics leading to EDGC’s birth into cancer diagnostics.
He explained how the concept of non-invasive prenatal (NIP) diagnosis was now being applied to cancer.
“In prenatal diagnosis, there is a small quantity of the baby's DNA floating around in the mother's blood. Similarly, when you get cancer, there are small amounts of DNA from the tumor tissue in the bloodstream,” said Lee. “We take the blood and perform very in-depth sequencing analysis to identify either the baby's DNA from the mother's DNA or cancer DNA from normal DNA."
Enabling early cancer detection
The core technology revolves around the company’s EpiCatch platform which is a patented low methylation concentration technology that utilizes selective amplification of DNA and next-generation sequencing (NGS) to discover epigenetic biomarkers found in cell-free DNA (cf-DNA), circulating tumor cells (CTC), and exosomes.
“As cancer cells divide and undergo apoptosis, cfDNA and circulating tumor DNA (ct-DNA) from cancer cells is released and circulated into the bloodstream. Oncocatch-E uses NGS to detect these cancer cell-derived DNA methylation signals,” explained Lee. “An AI algorithm then detects the presence of cancer based on methylation signals from a small amount of cancer cell-derived DNA.”
DNA methylation is a fundamental mechanism in epigenetics that plays a key role in regulating gene expression. Increased or decreased DNA methylation compared to normal cells is commonly observed in cancer cells, and is used as a marker for cancer diagnosis and treatment.
The test uses a restriction enzyme-based method to detect and quantify global genomic hypo-methylation. If cancer is detected, the algorithm identifies the specific cancer type and origin with high accuracy.
“Unlike late cancer screening where you can easily detect the origin of cancer through X-ray or CT imaging, this is difficult to identify as it’s a very small quantity in your blood but our developed test makes it possible,” said Lee. “Most of the news focuses on cancer treatment rather than diagnostics but to reduce cancer mortality the best approach is early detection as the rate of curing cancer reduces the later it is discovered.”
Making cancer screening more convenient
Acknowledging that irregular screening could still pose a problem, he highlighted Korea as one of the best countries to implement this as routine screening is regularly done due to the National Health Insurance Service (NHIS).
“With the exception of mammography, routine cancer screening with biomarkers is not that accurate so this tends to turn people off from doing the test,” said Lee.
At the same time, he noted that mammography is an inconvenient and painful process for most, and similarly, for men, colonoscopies for colon cancer screening are just as uncomfortable.
He further pointed out that most screening technology is for single-type cancers but there is no multi-cancer screening with just one blood draw with an accuracy greater than 90 percent such as EDGC's OncoCatch-E.
Recently, the company completed clinical validation studies to enable multi-cancer screening of four cancer types including breast cancer which was conducted at the Catholic University of Korea, Seoul St. Mary's Hospital, colon and gastric cancer at the Catholic University of Korea, Bucheon St. Mary's Hospital and lung cancer at Samsung Medical Center (SMC).
"The clinical validation is required to offer the test as a lab-developed test (LDT) for Clinical Laboratory Improvement Amendments (CLIA) in the U.S. It is a comparison of test results from known samples, done in the lab," explained Lee. "We are working on setting up labs in the U.S. and other countries but to start offering the service quickly to a global audience, we’ll use the Korean EDGC lab, which has a CLIA license and CAP accreditation to handle most of our global testing, including domestic samples," explained Lee.
The multi-cancer test is expected to launch in the U.S. and Korea later this year.
“Our goal is to be able to perform at least 10 different types of cancers with one drop of blood by next year as we can use the same pattern recognition to identify other cancers," he revealed.
Methylation changes also facilitating precision medicine
In this regard, he noted a recent melanoma cancer study which was successfully completed in Saint John's Cancer Institute, and is expected to issue a press release detailing the results this week. This Institute is part of the EDGC-led Epic-Catch consortium which aims to collaborate with domestic and international hospitals and research facilities for the discovery of epigenetic biomarkers.
“Although there is no limit to the number of cancers we can screen with this technology, we really need partners to help support the clinical trials so that the algorithm can train on the necessary data from cancer patients to identify the patterns and determine a specific cancer.”
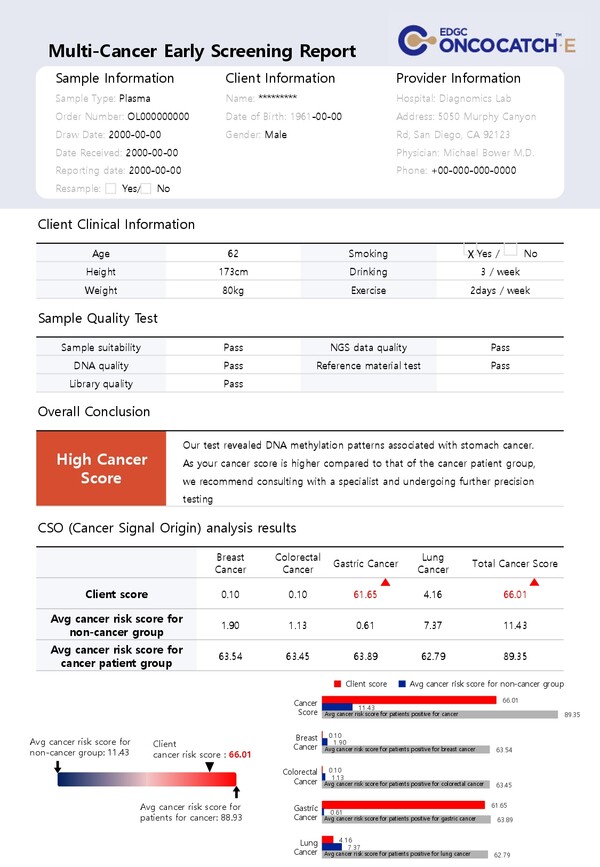
Everyone has cancer cells but each has a different rate of activation for these cancers, said Lee.
Accordingly, he pointed out that the scores generated by the AI algorithm also indicate your cancer risk. Thus, he advocated for the use of the platform in annual health screenings to enable continuous monitoring of cancer so that lifestyle habits can be adapted to minimize the cancer risk and even detect cancer before stage one.
“We are also trying to partner with pharmaceutical companies who are developing cancer drugs as patients tend to develop resistance to certain drugs,” he explained. “Mutations take longer times to develop but methylation changes develop quicker and are more likely to be the factor impacting the development of resistance to cancer drugs.”
Accordingly, Lee proposes to use the EpiCatch platform to identify biomarkers and make them into targeted therapies to enable a personalized medicine approach for patients with certain mutations or genetic variations that are not responding to the drug.
This is also possible for other diseases that are associated with methylation changes, he added.
Pointing out that tech companies like Amazon are making large investments in start-ups like Altos Lab in Silicon Valley, Lee mentioned the high value of epigenetic studies which also form the foundation of Altos Lab’s anti-aging technologies.
"Through our internal data of Alzheimer patients, we are investigating methylation patterns to determine whether the patient is at preclinical stages or might already have cognitive impairment," said Lee.
Although point-of-care diagnostics is a trend in the industry, he emphasized that the business was focused on being data-driven as the value of the platform revolves around the methylation screening of 65,000 patterns which needs to be performed in a laboratory setting.
“We already have a database consisting of gene pools from 500,000 Koreans and by further developing our legacy data, we will be able to follow patients’ disease histories through their methylation changes,” explained Lee. “We care for the data that we can collect from those patients to facilitate healthy lives and precision medicine.”
Initiating collaborations with Cancer X to enable early screening
The CEO also explained that several companies are offering liquid biopsy tests in Korea but the level of analysis differs.
First-generation liquid biopsy analyzes single biomarkers using DNA, or KRAS or EGFR-based mutations from your blood. Second-generation analysis includes multiple biomarkers from the DNA, and third-generation generation uses multiomics markers derived from DNA and epigenetics.
"EDGC is the only company offering fourth-generation liquid biopsy analysis using multiomics markers with multiplexing screening of epigenetics to identify the tumor signal of origin,” asserted Lee. “Even U.S. companies like Grail which has launched similar multi-cancer epigenetic studies are still far behind us.”
Though he expects the cost to be expensive as they are performing large-scale analysis of genes and it will take time to be covered by insurance, he highlighted the comparative cost benefit to single cancer screening tests.
Accordingly, he brought up the company’s recent decision to join the Cancer Moonshot project organized by Cancer X.
“We approached Cancer X with our early cancer screening program as we really need to work with multidisciplinary groups to implement it on national scales to everyone who can benefit,” said Lee. "At the recent Cancer X summit held in Washinton D.C. last week, we were the first to announce a collaboration with a Cancer X member. "
EDGC made an agreement with the City of Hope, a founding member of Cancer X and one of the largest national cancer centers in California, to commercialize the multi-cancer diagnostic. The CEO added that they are also working on a public-private partnership (PPP) with the Department of Health and Human Services in the U.S. through Cancer X for early cancer screening in the U.S.
Related articles
- EDGC launches liquid biopsy business in Vietnam
- [Reporter's Notebook] Korean companies bet on Cancer Moonshot but uncertainty looms
- EDGC aims to register liquid biopsy CDx on US Cancer Moonshot project
- Handok submits global phase 2/3 IND plan for biliary tract cancer drug candidate
- EDGC completes acquisition of US CLIA Lab
- EDGC gets US CLIA certification to ensure quality laboratory testing
- KRX suspends EDGC trading due to audit result controversy

