AstraZeneca's Soliris (eculizumab) has demonstrated its long-term efficacy and safety profile in a real-world study in Korean patients with paroxysmal nocturnal hemoglobinuria (PNH).
The October issue of the Journal of Korean Medical Science, an international medical journal of the Korean Medical Association, recently published 10-year real-world data of Soliris in Korean patients with PNH, titled “Long-Term Efficacy and Safety of Eculizumab in Patients With Paroxysmal Nocturnal Hemoglobinuria and High Disease Burden: Real-World Data From Korea.”
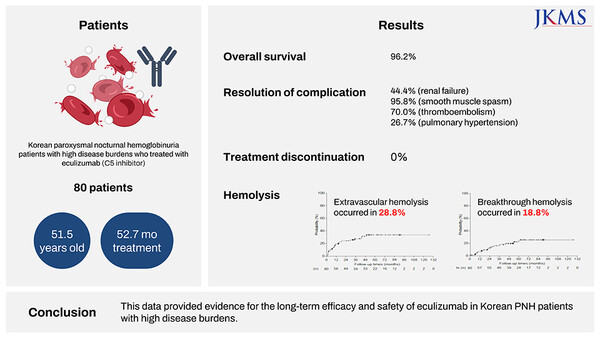
The study was a retrospective, multicenter observational study that evaluated the efficacy and safety of Soliris in 80 adult patients with PNH treated with Soliris at 14 medical institutions in Korea over 10 years from December 2009 to January 2020 based on data from the Health Insurance Review and Assessment Service (HIRA).
The primary endpoint was the investigator's assessment of drug efficacy, including the occurrence of intravascular hemolysis (IVH), disease-related complications and clinical events, improvement in anemia moderation and complications at the last follow-up, and clinical presentation. Additional endpoints included extravascular hemolysis (EVH), breakthrough hemolysis (BTH), and five-year survival.
Safety events included:
● Infections.
● Adverse events requiring discontinuation.
● Treatment-related fatal events that occurred up to three months after treatment with Soliris or the end of the study.
The median duration of treatment with Soliris for patients included in the study was 52.7 months.
The study found that despite patients experiencing severe symptoms before treatment, 96.3 percent were rated as having symptomatic improvement with Soliris in the drug efficacy assessment at the last follow-up. The five-year overall survival rate with Soliris was 96.2 percent, confirming the potential for long-term symptom-free survival.
Lactate dehydrogenase (LDH) levels, a key endpoint in paroxysmal nocturnal hemoglobinuria that indicates the extent of intravascular hemolysis improved to less than or equal to 1.5 times the upper limit of normal in more than 95 percent of patients at six months on Soliris, with improvement maintained through about eight years. In addition, 61.3 percent of patients achieved LDH in the normal range after treatment.
There was also an overall reduction in symptoms, such as fatigue, anemia, and dyspnea, which can impair patients' quality of life with PNH. Extravascular hemolysis was reported in 28.8 percent of patients, with nine of 23 receiving treatment. There were no significant changes in LDH due to extravasation. Sudden hemolysis was experienced by 18.8 percent of patients, similar to the incidence seen in previous studies.
Safety evaluations showed that more than half (55.7 percent) of patients did not report any complications during treatment, including thromboembolic events, pulmonary hypertension, or renal failure. No patients discontinued treatment due to lack of response or loss of response to Soliris during treatment, indicating that it was well tolerated over the long term. Two deaths were reported but were not considered related to treatment.
"This study confirms the long-term efficacy and safety of Soliris, which celebrates its 10th anniversary this year, in patients with paroxysmal nocturnal hemoglobinuria in real-world clinical settings," said Kim Chul-woong, Business Unit Director for Rare Diseases at AstraZeneca Korea. "As a company with both Soliris and Ultomiris for paroxysmal nocturnal hemoglobinuria, we will strive to strengthen our market leadership through continuous research to improve the treatment landscape."
Soliris, the first treatment for PNH approved in Korea in 2010, is a complement inhibitor that binds to the C5 protein and inhibits the complement chain reaction at the end of the nervous system.
It has received insurance coverage for PNH since October 2012, with the addition of atypical Hemolytic Uremic Syndrome (aHUS) in 2016 and Neuromyelitis Optica Spectrum Disorder (NMOSD) in 2021, and is used for intractable rare diseases.

