Lunit said Tuesday that the result of applying the AI-based Lunit Scope as an exploratory biomarker in phase 3 immunotherapy-chemotherapy combination study in patients with genetically altered non-small cell lung cancer (NSCLC) has been published in the Journal of Clinical Oncology (JCO).
JCO is the world's most prestigious international journal in the field of oncology, published by the American Society of Clinical Oncology (ASCO). Lunit has published its latest research on AI biomarkers in JCO this and last year. Professors Ahn Myung-ju and Park Se-hoon of the Department of Hematology/Oncology at Samsung Medical Center led the research.
The research team demonstrated the clinical efficacy of combining immunotherapies, such as Tecentriq (atezolizumab) and Avastin (bevacizumab), with chemotherapy in patients with EGFR and ALK mutations in NSCLC.
The researchers randomized 228 patients -- 215 with EGFR mutations and 13 with ALK mutations -- in a 2:1 ratio at 16 medical institutions in Korea.
The researchers divided the patients into two groups to compare the clinical efficacy of atezolizumab, bevacizumab, paclitaxel, and carboplatin in one arm (the ABCP arm) and pemetrexed and carboplatin or cisplatin in the other arm (the PC arm).
The results showed that median progression-free survival (mPFS), the study's primary endpoint, was significantly longer in the ABCP arm at 8.48 months compared to 5.62 months in the PC arm. The objective response rate (ORR) was 69.5 percent in the ABCP arm, compared to 41.9 percent in the PC arm.
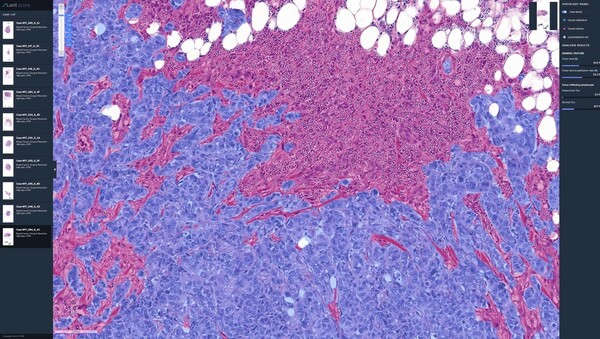
The researchers also analyzed the spatial distribution of tumor-infiltrating lymphocytes (TILs), an immune cell in cancer tissue. They evaluated their immunophenotype (IP) using Lunit Scope to predict immunotherapy prognosis.
The results showed that PFS in the group with TIL expression of 20 percent or less was not significantly different, with 8.28 months in the ABCP group and 6.93 months in the PC group. In contrast, the PFS in the group with TIL expression of 20 percent or higher significantly differed at 12.91 months in the ABCP arm and 4.86 months in the PC arm.
"These results suggest that Lunit Scope can accurately detect immune activity based on the distribution of TILs, which may be useful for selecting patients who may benefit from the combination of immunotherapies compared to conventional chemotherapy," Lunit said.
Lunit CEO Suh Beom-seok said, "This study was recently selected as a 'Late-breaking Abstract' at the European Society for Medical Oncology (ESMO 2023), one of the world's three major cancer conferences, focusing the attention of academia and industry on AI biomarkers."
Especially since this study is a clinical trial that has failed several times in the past by global pharmaceutical giants, the company expects further collaborative activities, such as more research on this and the development of a companion diagnostic model with global pharmaceutical companies to obtain approval from the U.S. FDA, he added.
Related articles
- Lunit, 1st Korean firm to join Saudi Vision 2030 Healthcare Sandbox, aims AI-powered cancer care
- [ESMO 2023] Lunit, Medpacto, TiumBio present research results at ESMO 2023
- Lunit, UK hospital to launch prospective AI study in symptomatic breast clinic setting
- Lunit AI finds its place in European breast cancer screening system
- Lunit agrees with MD Anderson on AI-based research of immunotherapies’ efficacy

