The Ministry of Food and Drug Safety (MFDS) has announced that South Korea has become one of three nations, alongside Switzerland and Singapore, officially listed on the World Health Organization's (WHO) first-round roster of WHO Listed Authorities (WLA).
The WLA system is a recent initiative by WHO, aimed at cataloging regulatory authorities based on their exceptional regulatory systems and operational capabilities. It is set to replace the existing list of Stringent Regulatory Authorities (SRA), which was primarily established to facilitate pharmaceutical procurement by UN agencies like UNICEF.
In 2015, WHO designated 36 countries, including the U.S., Japan, the U.K., France, Germany, Croatia, Latvia, and Malta, as SRAs.
Despite being an active member of the International Council for Harmonisation (ICH) since 2016, Korea was not included in the SRA roster.
Last year, WHO rolled out a pilot program for the WLA system and temporarily listed the original SRA countries, along with the European Medicines Agency, as interim WLA members.
These countries are expected to undergo a formal or streamlined risk-based evaluation within the next five years to secure a permanent spot on the WLA list. Not having been listed as an SRA, Korea took proactive measures and began its preparations for the WLA listing in 2021.
The WLA inclusion means that domestic pharmaceutical companies in South Korea will likely be granted exceptions to the WHO Quality Certification (PQ) when bidding for drug procurement by UN subsidiary organizations.
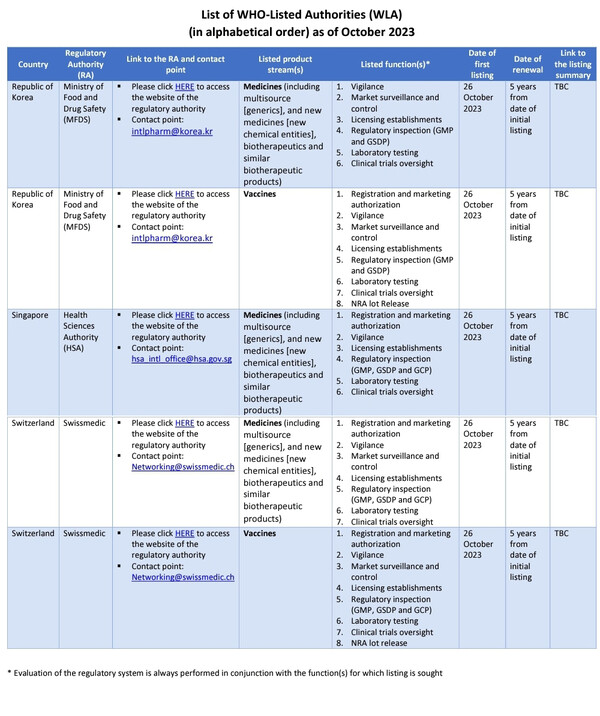
Unlike Singapore, which only secured WLA listing for pharmaceuticals, both Korea and Switzerland have achieved the privilege in two domains -- vaccines and pharmaceuticals.
Specifically, the MFDS has been certified for eight functions in the vaccine sector, which encompass registration and marketing authorization, vigilance, market surveillance and control, licensing establishments, regulatory inspection (GMP and GSDP), laboratory testing, clinical trials oversight, and national regulatory authority (NRA) lot release.
In the realm of pharmaceuticals, six functions were certified, excluding registration and marketing authorization, and NRA lot release. However, the MFDS is currently finalizing an assessment procedure intending to list the marketing authorization function for pharmaceuticals.
"The WLA listing of MFDS underscores the excellence of our government's regulatory system in the fields of pharmaceuticals and vaccines," Food and Drug Safety Minister Oh Yu-kyoung said. "It reaffirms, on a global scale, that our domestic pharmaceutical and vaccine manufacturers produce trustworthy products."

