A clinical trial led by a Korean researcher for treating advanced gastric cancer has changed global standards and provided new strategies for designing successful umbrella trials.
It was a good example that highlighted the top-notch research capabilities of Korean gastric cancer experts.
The American Society of Clinical Oncology's Journal of Clinical Oncology (JCO) recently published the results of the “K-Umbrella” gastric cancer study, an investigator-initiated clinical trial led by Dr. Rha Sun-young, a professor of the Department of Medical Oncology at Yonsei Cancer Center.

The K-Umbrella Study is an umbrella clinical trial for advanced gastric cancer patients in Korea led by Professor Rha, also chairperson of the Gastric Cancer Section of the Korean Cancer Study Group (KCSG). It has been in the spotlight since the first data was released at the American Society of Clinical Oncology 2022 Annual Meeting (ASCO 2022).
Now, the trial's value has won additional recognition among oncology experts worldwide with its publication in JCO, the world's most prestigious journal in the field of oncology.
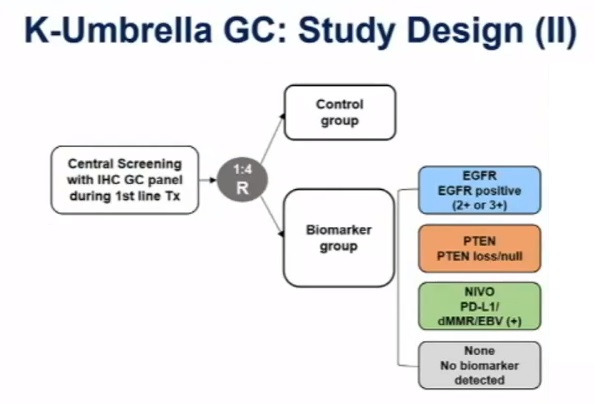
The study compared the efficacy and safety of biomarker-based drug therapy with conventional standard of care in the second-line treatment of advanced gastric cancer, using a novel and unprecedented umbrella trial design.
The trial was divided into multiple cohorts of integrated biomarker groups and a control group using conventional standard of care (SOC).
"Multi-arm studies are challenging because they require a control group for each arm, which doubles the number of patients," Professor Rha said. "In this study, we tried a new design incorporating multiple cohorts based on biomarkers with a common control group as the main focus."
The biomarker group was divided into four cohorts: patients with EGFR 2+/3+ (EGFR cohort) were randomized to afatinib, a broad-spectrum ERBB inhibitor; patients with PTEN loss/deletion (PTEN cohort) were randomized to GSK2636771, a PIK3Cβ inhibitor; PD-L1+, dMMR/MSI-H or EBV-associated (NIVO cohort) were treated with the anti-PD-1 inhibitor nivolumab in combination with paclitaxel; and the remaining patients without pre-defined biomarkers (NONE cohort) were treated with standard of care (SOC).
Initially, 722 patients were screened for the study, but only 318 were included. During the study, the standard of care for second-line treatment of advanced gastric cancer changed from paclitaxel alone to Cyramza (ramucirumab) plus paclitaxel, which meant that the control group had to be changed to match the standard of care, significantly reducing the sample size.
That was also the main reason the study found no significant difference in the median progression-free survival (mPFS 3.7 months vs. 4.0 months) and the median overall survival (mOS 8.6 months vs. 8.7 months) between the biomarker and control groups.
"While these findings do not have immediate implications for current clinical practice, they provide important principles for the design of successful large-scale biomarker-driven trials in patients with advanced gastric cancer," said Dr. Andrew H. Ko, JCO associate editor and professor of medicine at the University of California, San Francisco, who reviewed the study.
Professor Rha's contributions to treating advanced gastric cancer did not stop there. She led the successful KEYNOTE-859 study that approved Keytruda (pembrolizumab) as the new standard of care for the first-line treatment of HER2-negative patients.
In KEYNOTE-859, Keytruda demonstrated a statistically significant improvement in overall survival (OS) in combination with fluoropyrimidine and platinum-based chemotherapy compared to chemotherapy alone and is pursuing indication expansion in the U.S. and Europe.
Most recently, the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended approval of Keytruda in combination with fluoropyrimidine and platinum-based chemotherapy for the "first-line treatment of locally advanced or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma that is PD-L1-positive (CPS ≥1) and inoperable," indicating that European approval is imminent.
As the trial's principal investigator, Professor Rha successfully demonstrated clinical improvement with the combination and was a key contributor to the expansion of Keytruda's indications.
The results of the KEYNOTE-859 study were also published in the prestigious international journal, The Lancet Oncology, and have been recognized by researchers worldwide.
All this shows Professor Rah has successfully led advanced gastric cancer from licensed clinical trials to investigator-initiated clinical trials, further cementing Korea's pioneering status in the treatment of gastric cancer, according to medical community insiders.

