Researchers at the Korea Advanced Institute of Science and Technology (KAIST), in collaboration with the Korea Basic Science Institute (KBSI), have discovered a cellular protein that accelerates the toxicity leading to Alzheimer's disease, revealing a novel pathological network associated with the disease.
Alzheimer's disease is the most representative neurodegenerative disorder, causing memory loss and cognitive decline. Despite the increasing prevalence of Alzheimer's disease, the exact cause remains unclear, hindering the development of effective treatments.
Pathological hallmarks of Alzheimer's in the brain include the accumulation of senile plaques predominantly made of amyloid-beta peptide aggregates. These aggregates bind to substances within cells, leading to cellular damage.
The correlation between these aggregates and cell death has been the subject of extensive research, but direct interactions between amyloid-beta and cellular death inducers are not well understood.
Recent FDA-approved medications for Alzheimer's target these amyloid-beta aggregates to prevent cellular damage. However, side effects have limited their use, prompting researchers to explore new directions in drug development.
As a result, the interdisciplinary research team, led by Professor Lim Mi-hee, Han Jin-ju, and Baik Mu-hyun, at KAIST, Lee Young-ho at KBSI, and Lee Da-yong at Korea Research Institute of Bioscience and Biotechnology (KRIBB), have identified a cellular protein that enhances the toxicity of factors inducing Alzheimer's disease, paving the way for new insights into the disease's mechanisms.
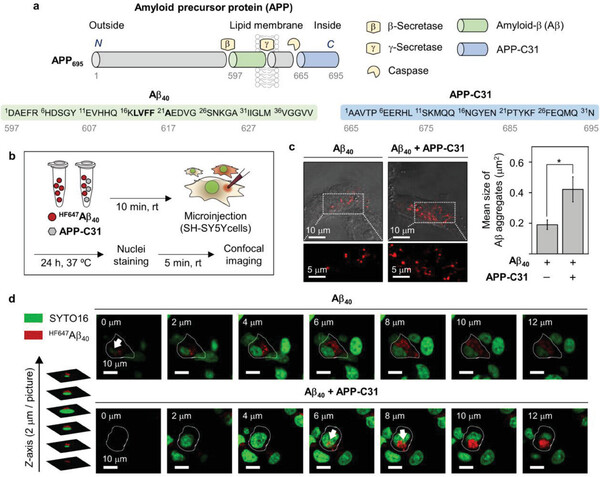
Professor Lim's team has demonstrated that a protein known as the amyloid precursor C-terminal fragment (C-CTF), which is overexpressed in Alzheimer's and induces unknown neuronal cell death, binds with amyloid-beta and metal-amyloid-beta complexes to enhance aggregation and toxicity.
The findings suggest that the C-CTF alone or in complex with amyloid-beta could serve as a new biomarker for Alzheimer's and potentially as a target for drug development.
The team employed intracellular protein microinjection techniques to investigate the role of C-CTF in promoting amyloid-beta aggregation within cells. They found that C-CTF worsens cell death, neuronal damage, and inflammatory responses linked to amyloid-beta in neurons and rodent brains.
"This research result has great significance in the discovery of previously unknown in vivo amyloid-beta aggregation and toxicity promoters in Alzheimer's disease," Lim said. "This research achievement suggests new biomarkers and therapeutic targets.".
The research results were published in Advanced Science.

