Samil Pharmaceutical said Monday that its U.S. partner firm Biosplice Therapeutics confirmed the efficacy of Lorecivivint, a drug candidate for knee osteoarthritis, in a phase 3 study called OA-07. These results were recently revealed during the American College of Rheumatology (ACR) meeting held in San Diego, California, on Nov. 13.
Biosplice Therapeutics developed Lorecivivint, and in March 2021, Samil Pharmaceutical obtained exclusive rights to license and market the drug in Korea.
Lorecivivint is a Cdc-Like Kinase (CLK)/Dual specificity tyrosine-phosphorylation-regulated kinase (DYRK) inhibitor. It has a mechanism of action that modulates Wingless and int (Wnt) signaling and is being developed as a disease-modifying osteoarthritis drug (DMOAD).
OA-07 is an extension study in 276 patients who completed the phase 3 OA-11 study in the U.S. The study aimed to assess Lorecivivint when administered annually over a three-year period.
In Year 1 of OA-07, the study arm received 0.07 mg of Lorecivivint, while the control arm received a placebo. In Year 2 of the OA-07 trial, the treatment and control groups received 0.07 mg of Lorecivivint. While the OA-07 trial was ongoing, the results of the previous trial, OA-11, remained double-blind.
Clinical results showed a minimum joint space width (mJSW) of -0.06 mm in the treatment group received Lorecivivint 0.07 mg once a year for three years and a mJSW of -0.21 mm in the placebo control group.
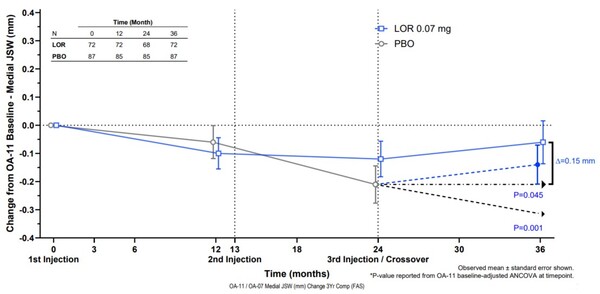
Assuming the control group received a placebo for 36 months, Samil said the difference in mJSW between the trial and placebo groups was 0.26 mm, which was clinically and statistically significant.
The mJSW is measured by x-ray, showing knee osteoarthritis progression based on its width. A smaller mJSW indicates more severe osteoarthritis progression, while an increased mJSW indicates improved osteoarthritis.
"These results indicate that repeated injections of Lorecivivint may provide both structural changes and symptomatic benefits," said Yusuf Yazici, a researcher of Biosplice Therapeutics, who presented the phase 3 results. "We are excited to continue to study Lorecivivint as a treatment option for knee osteoarthritis."
"The clinical results are significant as they confirm long-term safety and efficacy. It is expected to become a meaningful new drug portfolio for Samil Pharmaceutical, which has exclusive domestic rights," a Samil official said.

