Roche's drug Columvi (glofitamab), designed to treat diffuse large B cell lymphoma (DLBCL), has received approval in Korea.
The Ministry of Food and Drug Safety (MFDS) said Thursday that it gave the go-ahead to the Swiss-based pharma’s orphan drug.
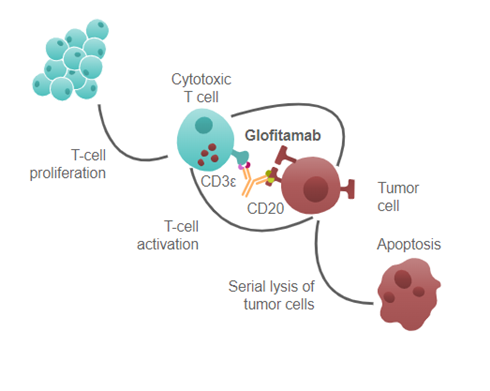
Columvi is a CD20/CD3 bispecific antibody that binds to CD3 on the surface of T cells and CD20 on the surface of B cells. It was approved for treating patients with relapsed or refractory diffuse large B-cell lymphoma after two or more systemic therapies.
The drug binds to CD3, which activates T-cells, and binds to CD20, which positions B-cells next to the activated T-cells and induces lysis of the B-cells.
Roche Korea also affirmed Columvi’s approval on Friday, stressing that it is the first bispecific antibody B-cell lymphoma treatment.
The company explained that Columvi is available as an off-the-shelf drug, allowing patients to get treatment immediately upon diagnosis without waiting for the drug to be manufactured. The treatment period is fixed at about 8.3 months (up to 12 cycles). Patients can get treatment at nationwide hospitals that have the drug by visiting hospitals every three weeks without hospitalization.
The approval is based on the multi-institutional, open-label phase 1 and 2 NP30179 clinical study. In the study, the primary endpoint of complete response (CR) reached 40 percent (61/155 [95 percent CI: 32-48]), and the secondary endpoint of overall response rate (ORR) was 52 percent (80/155 [95 percent CI: 44-60]) in patients treated with Columvi monotherapy for a fixed duration of 8.3 months (up to 12 cycles).
The median time to complete remission with Columvi was 42 days [95 percent CI: 42-44], demonstrating a rapid response to treatment. Among patients who achieved a complete response, the median duration of response was 26.9 months (18.4-NR), with 67 percent of patients maintaining a complete response at 18 months. The most common adverse event was cytokine release syndrome (CRS), which occurred in 97 of 154 patients (63 percent) but was mostly mild.
"DLBCL is a rapidly progressive aggressive lymphoma with a poor prognosis at advanced stages, requiring effective treatment without delay. However, there is an unmet need for new therapeutic options as it is difficult to start treatment immediately or cytotoxic chemotherapy is administered indefinitely until the tumor progresses," said Professor Yoon Sung-soo of the Department of Hematology/Oncology at Seoul National University Hospital.
Roche Korea President and General Manager Nic Horridge said, "We are pleased to be able to offer new and innovative treatment options to patients with relapsed or refractory DLBCL who have failed multiple therapies.
Roche Korea will continue to do its best to deliver therapeutic innovations that address the unmet needs of patients with blood cancers in Korea through Columvi and other new blood cancer medicines, including Polivy, the first first-line treatment for DLBCL in 20 years, and Lunsumio, the MFDS’s GIFT No. 1 treatment for relapsed or refractory vesicular lymphoma, Horridge added.
DLBCL is a disease in which the body's protective B cells grow or multiply uncontrollably, accounting for about 40 percent of non-Hodgkin lymphoma (NHL). It is an aggressive subtype of the disease that progresses rapidly, requiring treatment to be initiated quickly after diagnosis, and is characterized by a rapidly deteriorating prognosis as the number of lines of treatment increases. The number of patients in Korea stood at 12,910 in 2022, up 31.8 percent from 9,791 in 2017.

