AstraZeneca Korea has received marketing authorization for Tezspire (tezepelumab), a treatment for severe asthma in Korea.
The Ministry of Food and Drug Safety (MFDS) approved Tezpire on Thursday.
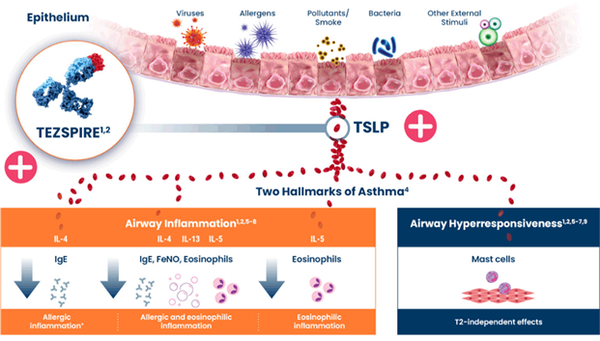
Tezspire is an anti-TSLP monoclonal antibody that binds to thymic stromal lymphopoietin (TSLP), which causes airway inflammation and blocks TSLP-induced inflammation.
TSLP is a cytokine, a signaling molecule that induces an immune response and is expressed by epithelial cells in the thymus gland, lungs, and skin in response to stimulation by foreign antigens.
Tezspire's approved indication in Korea is for patients 12 and older with severe asthma not adequately controlled by conventional maintenance therapy. It is the first anti-TSLP therapy approved in Korea while existing treatments for severe asthma target IgE or IL-5 in mast cells.
The drug won approval from the U.S. Food and Drug Administration in December 2021 for treating severe asthma.
According to the PATHFINDER clinical study that led to the approval of Tezspire, the drug effectively reduced asthma exacerbations, improved lung function, and improved quality of life in patients with severe asthma. It also reduced the exacerbations of asthma regardless of blood eosinophil counts.
"We will continue to do our best to ensure that therapeutic drugs with sufficiently confirmed safety and effectiveness are quickly supplied based on our expertise in regulatory science," the ministry said.

