A new targeted therapy demonstrated safety and effectiveness in ROS1 fusion-positive non-small-cell lung cancer (NSCLC), a study showed.

A research team, led by Professor Cho Byoung-chul at Yonsei Cancer Center, published the results of a study on the effectiveness and safety of repotrectinib in patients with ROS1 fusion-positive NSCLC who were either previously untreated or resistant to existing targeted therapies.
The findings have been published in the international journal New England Journal of Medicine (IF 176.082). Notably, Professor Cho is the first Korean to publish a study in the NEJM as a corresponding author in oncology.
ROS1 infusion-positive NSCLC constitutes 2 percent of all lung cancers. The established standard of care involves the utilization of targeted therapies specifically designed to address the mutated gene. Among the primary targeted therapies are crizotinib and entrectinib. These therapies have exhibited an objective response rate (ORR) of 70 percent, indicating the percentage of patients experiencing a reduction in tumor size. Additionally, progression-free survival (PFS), a crucial metric measuring the duration a patient survives without disease progression, ranges from 15 to 19 months. These ORR and PFS figures serve as key indicators of the success of cancer drug treatments.
Generally, when resistance to targeted therapy develops, there are limited effective therapeutic alternatives to cytotoxic chemotherapy. Notably, both crizotinib and entrectinib, the primary targeted therapies, exhibit poor brain penetration. Consequently, approximately 70 percent of patients with resistance experience the development or exacerbation of brain metastases.
In response to these challenges, Cho spearheaded the multinational phase 1-2 TRIDENT-1 study. This research showcased the efficacy and safety of repotrectinib, a next-generation ROS1-targeted therapy, offering a potential solution to address the limitations associated with existing treatments.
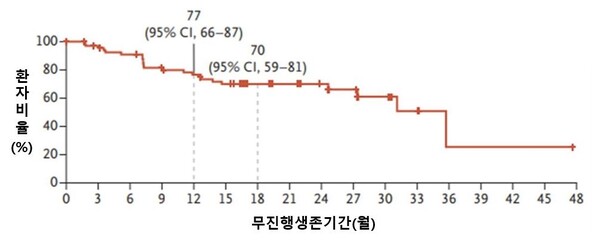
In the initial cohort of 71 patients treated with the targeted therapy for the first time, the ORR marked 79 percent, and PFS, 35.7 months. The PFS in the targeted therapy group nearly doubled, compared to previous targeted therapies.
Furthermore, the study highlighted the effectiveness of the new targeted therapy in individuals resistant to prior targeted therapies. Among the 56 patients with resistance, there was a noteworthy ORR of 38 percent and a PFS of nine months. Notably, within this group, 17 patients who acquired a resistance mutation (G2032R) demonstrated an even more substantial ORR of 59 percent and a PFS of 9.2 months.
Repotrectinib demonstrated strong efficacy also in patients with brain metastases, specifically achieving an impressive intracranial response rate of 89 percent in patients who had not received prior targeted therapy.
The most frequently reported adverse effect of repotrectinib was dizziness, although the majority of these side effects were deemed manageable.
"Repotrectinib represents a next-generation targeted therapy that surpasses the limitations of existing ROS1-targeted treatments," said Professor Cho, who is leading the Lung Cancer Center at Yonsei Cancer Center. Cho has been overseeing the study since its preclinical development. "It introduces new therapeutic possibilities as a first-line agent for ROS1 fusion-positive NSCLC and offers renewed optimism for individuals resistant to prior targeted therapies."
Subsequent to these findings, the U.S. FDA granted approval for repotrectinib's use in metastatic ROS1-positive NSCLC in November, he added.

