The National Assembly has responded to the voices of patients calling for including triple-negative breast cancer drug Trodelvy (sacituzumab govitecan) in health insurance.
The public petition, signed by 50,000 people, was referred to the Health and Welfare Committee, a relevant Assembly standing committee.
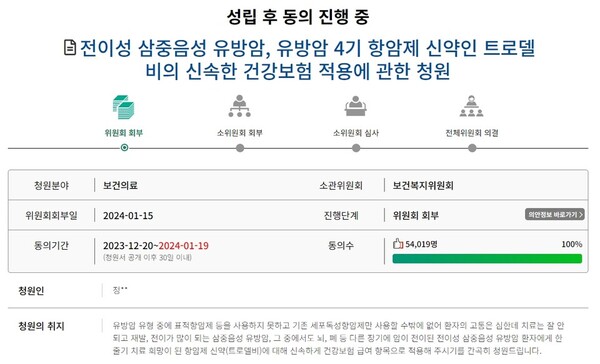
The National Assembly said Monday that the "Petition for prompt health insurance coverage of Trodelvy, a new drug for metastatic triple-negative breast cancer and stage IV breast cancer," has been referred to its Health and Welfare Committee for discussion. The petition has gathered 50,000 signatures with five days to go before the end of the deadline.
Triple-negative breast cancer is breast cancer in which the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER2) are all negative and are associated with a higher risk of metastasis and recurrence than other breast cancer subtypes. Marked by a poor prognosis for treatment, it often metastasizes to the brain (30 percent) or lungs (40 percent). The five-year survival rate is only 12 percent for triple-negative breast cancer, compared to 30 percent for other types.
Trodelvy demonstrated significant clinical utility in the phase 3 ASCENT study, a licensed clinical trial. In patients with unresectable locally advanced or metastatic triple-negative breast cancer who had received two or more prior systemic therapies, at least one of which was for metastatic disease, Trodelvy reduced the risk of death by 49 percent (11.8 months vs. 6.9 months) and improved progression-free survival by 57 percent (4.8 months vs. 1.7 months) compared to single-agent chemotherapy with Treatment of Physician's Choice (TPC).
These benefits were consistent regardless of the presence of brain metastases.
Because of these efficacies, Trodelvy has become a "ray of hope" for people with metastatic triple-negative breast cancer.
"My wife was diagnosed with triple-negative breast cancer in 2010 and was cured after five years through surgery, chemotherapy, and radiotherapy. However, in 2021, she developed a third triple-negative breast cancer, which has spread to both lungs," said a petitioner, the husband of a patient.
"After more than two years of chemotherapy, the cancer metastasized to her bones, kidneys, and then to her brain, and she received radiation treatment, but the cancer remains," the petitioner said. "In desperation, we purchased Trodelvy and injected it four times, but it was difficult to afford the drug price of 22 million won ($16,424).”
The patient received Trodelvy at a university hospital where the drug is available, but it is not yet covered by health insurance, so it costs 5.3 million won for one injection. The petitioner explained that the patient has to take three injections a month, which costs about 16 million won a month and nearly 200 million won a year.
"For patients with metastatic triple-negative breast cancer, other anticancer drugs are ineffective, and Trodelvy is almost the only treatment. I requested that health insurance be applied quickly so we can have the last hope, the injection," the petitioner said.
Trodelvy, which has emerged as a new treatment option for recurrent or metastatic triple-negative breast cancer, passed the first hurdle to insurance coverage last November. The Health Insurance Review and Assessment Service's Cancer Disease Review Committee set the reimbursement standard, but more obstacles remain before getting insurance benefits.
To receive health insurance coverage, Trodelvy must pass the Pharmaceutical Reimbursement Evaluation Committee, negotiate drug prices with the National Health Insurance Service, and pass the Health Insurance Policy Deliberation Committee.
To help ease the burden of treatment costs for patients, Gilead Sciences Korea supports a portion of the drug's cost for patients who have been prescribed the drug without coverage.

