Precision Biosensor, a Kosdaq-listed clinical diagnostics company, said Tuesday that it received marketing approval for two thyroid function hormone test reagents, Exdia TSH and Exdia fT4, from the Ministry of Food and Drug Safety (MFDS).
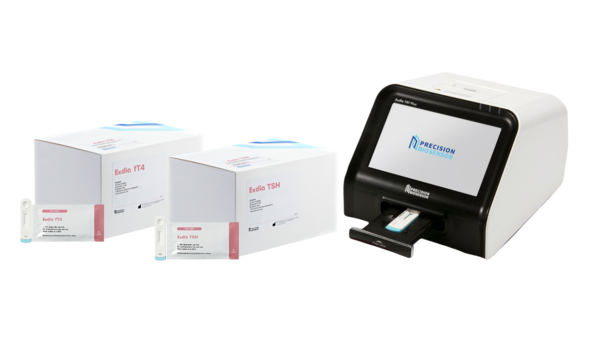
Exdia TSH and Exdia fT4 can be easily used in hospitals to obtain diagnostic results within 10 to 15 minutes using a dedicated test machine.
With this clearance, Precision Biosensors will expand the range of products tested with its clinical diagnostics device, Exdia TRF, from cardiovascular and infectious diseases to hormonal products, accelerating its entry into the domestic diagnostic market.
Thyroid hormones are the primary endocrine hormones that help regulate metabolism and body temperature
Major diseases related to thyroid hormone abnormalities include hypothyroidism and hyperthyroidism, representing symptoms such as indigestion and fatigue, which are often difficult to diagnose through clinical examination alone, demanding the need for blood tests.
According to the Health Insurance Review and Assessment Service (HIRA), the number of diagnoses related to thyroid function in Korea is 26.6 million in 2022, up from 15.6 million in 2018.
With this approval, Precision Biosensor will actively promote the expansion of its immunodiagnostic platform, Exdia TRF Plus testers, along with cardiovascular and infectious diagnostic cartridges that have already completed domestic licensing.
Precision Biosensors's immunodiagnostic platform Exdia TRF Plus, which is smaller than an A4 sheet of paper, has the advantage of being easy to install in hospitals.
"Our immunodiagnostic products have been steadily expanding sales in global markets such as Europe thanks to the accuracy of test results and ease of use," said Kim Han-shin, CEO of Precision Biosensors. "By utilizing our clinical chemistry and immunodiagnostic platform to integrate diagnosis, monitoring, and prescription of diseases, we plan to expand its use to chronic disease care in the future."

