Daewoong Pharmaceutical's Fexuclue is expanding globally as fast as it is effective.
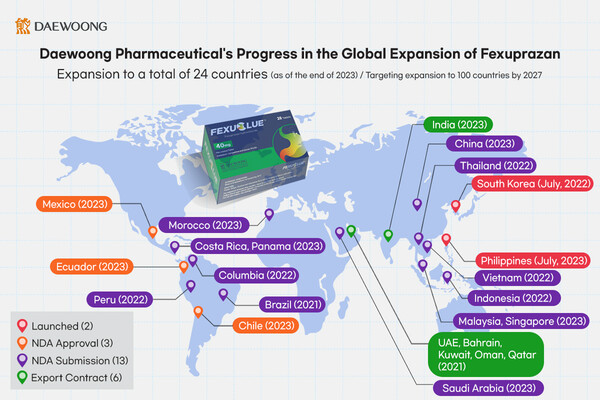
Daewoong said Friday that its gastroesophageal reflux disease (GERD) treatment, Fexuclue, has entered or is about to enter the market in 24 countries, including Korea, in just one year and six months since its launch.
As of February 2024, Fexuclue has entered two countries -- Korea and the Philippines. It has won approval in three Latin American countries -- Mexico, Ecuador, and Chile – and is preparing for local launches this year. In addition, it has applied for marketing authorization from 13 countries, including China and Saudi Arabia, has applied for marketing authorizations.
The total number of countries is 24, including six countries with export contracts, such as India and the United Arab Emirates.
The global anti-ulcer drug market is about 21 trillion won ($15.7 billion). The market size of the 24 countries that have introduced or are in the process of introducing Fexuclue is 8.4 trillion won, accounting for about 40 percent of the global market.
If the expansion into 24 countries is completed as planned, Daewoong's Fexuclue will become the first new drug launched globally by a Korean pharmaceutical company.
Industry observers say Daewoong's goal of "applying for approval in 30 countries in 2025 and entering 100 countries in 2027," as it promised when it launched Fexuclue, will be easily achieved. Daewoong also expects Fexuclue to drive its “one trillion won from one product” sales strategy applied to its self-developed drugs.
"There have been cases where homegrown new drugs have been signed into multinational contracts through global deals. However, it was difficult to label them as blockbusters because they failed to get licenses," a Daewoong official said. "Fexuclue has learned from past examples, established a strategy for domestic and international development from the beginning of development, and thoroughly verified the countries where actual development and sales can be carried out."
As a result, it will have entered 24 countries globally within one year and six months after launching in Korea and has applied for item licenses in 18 of them, the highest level among domestic new drugs in terms of global expansion speed, the official added.
Daewoong Pharmaceutical is further accelerating its global market expansion by applying for product licenses in 30 countries in 25 years and entering 100 countries in 27 years. This year, the company plans to increase the number of applications to 25 countries and expand the number of item licenses to more than six countries.
Fexucluse, Korea's 34th new drug, is a next-generation P-CAB-based GERD treatment that improves the shortcomings of PPI drugs, such as slow onset of action and dietary effects. It has the longest half-life of up to nine hours among GERD medicines, so it has a long-lasting effect and is effective in improving symptoms caused by nighttime acid secretion.
In addition, it is highly convenient to take only one tablet once daily regardless of whether you eat. It has strengths, including rapid onset of effect, rapid and excellent symptom improvement, improvement of symptoms caused by nocturnal acid secretion, ease of administration, low drug interactions, and consistency of efficacy.
"In addition to steep sales growth in Korea, 2023 was a year of tangible achievements globally, including our entry into India, the world's fourth-largest anti-ulcer drug market," Daewoong Pharmaceutical Vice President Park Sung-soo said. "We will grow Fexuclue into a global blockbuster in the GERD treatment market and accelerate our efforts.”
Meanwhile, Daewoong Pharmaceutical is actively pursuing global expansion under the “3E Global Super Gap Strategy.” It plans to realize 1 trillion won products for the first time in Korea through rapid global item approval (Efficiency), simultaneous expansion of new drug lineup (Extension), and overwhelming production excellence (Excellence) at the only four-stage smart factory in Korea that introduces AI.
Related articles
- Daewoong Pharmaceutical's operating profit hits record high in 2023 thanks to new drugs, BTX
- [CPHI 2023] Why Daewoong is confident of export competitiveness of P-CAB drugs
- Daewoong preprocessed '800 million compounds' and turned them into DB
- Korean pharmas urge MFDS to enhance export opporunities through international cooperation
- Daewoong's new co-CEO Park Seong-soo vows to achieve ₩1 tril. operating profit
- Daewoong, Chong Kun Dang to co-market GERD drug Fexuclue
- Fexuclue sales soar 57% in Q1, boosting Daewoong's GERD market growth

